
(a)
Interpretation:
Bond-line structure for the given set of amino acids in zwitterionic form need to be drawn.
Concept introduction:
Bond-line structure is the representation of organic structural formulas in a shorthand manner. In this case only the bond between carbon and other atoms are shown except hydrogen. It is understood that all the remaining valency to be filled by hydrogen. Apart from carbon atoms the other atoms are shown along with hydrogen.
Zwitterion is the one which has the positively and negatively charged groups separately. Zwitterion is found at the pH which is the isoelectric point.
To draw : To draw bond-line structure for L-valine
(a)
Answer to Problem 40PP
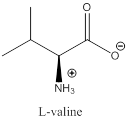
The bond-line structure for (a) and its zwitterion form is
Explanation of Solution
Linear formula for L-valine
The linear formula for L-valine is
Draw bonds between the atoms except hydrogen
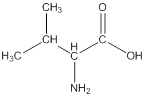
The structure from the linear formula is drawn as shown above. Considering the valency of carbon to be four, the structure is drawn.
Remove the carbons that are explicitly shown
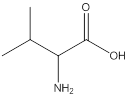
The carbon atoms are ignored from being explicitly shown as it is understood that the carbon and hydrogen atoms are not shown in bond-line structure.
Assign configuration
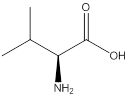
As in the name of the problem statement it is said L-valine, the chiral center must have “S” configuration and the same is shown in the above structure.
Zwitterion
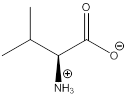
Zwitterion is one of the form in which the charge is separated and this is formed at a particular pH known as isoelectric point.
(b)
Interpretation:
Bond-line structure for the given set of amino acids in zwitterionic form need to be drawn.
Concept introduction:
Bond-line structure is the representation of organic structural formulas in a shorthand manner. In this case only the bond between carbon and other atoms are shown except hydrogen. It is understood that all the remaining valency to be filled by hydrogen. Apart from carbon atoms the other atoms are shown along with hydrogen.
Zwitterion is the one which has the positively and negatively charged groups separately. Zwitterion is found at the pH which is the isoelectric point.
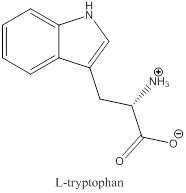
To draw : To draw bond-line structure for L-tryptophan
(b)
Answer to Problem 40PP
The bond-line structure for (b) and its zwitterion form is
Explanation of Solution
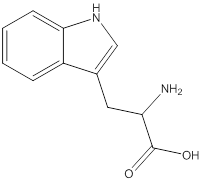
Linear formula for L-tryptophan
The linear formula for L-tryptophan is
Draw bonds between the atoms except hydrogen
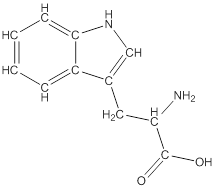
The structure from the linear formula is drawn as shown above. Considering the valency of carbon to be four, the structure is drawn. The benzopyroole ring is the one which is represented as
Remove the carbons that are explicitly shown
The carbon atoms are ignored from being explicitly shown as it is understood that the carbon and hydrogen atoms are not shown in bond-line structure.
Assign configuration
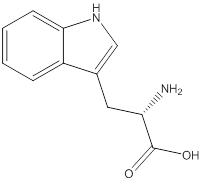
As in the name of the problem statement it is said L-tryptophan, the chiral center must have “S” configuration and the same is shown in the above structure.
Zwitterion
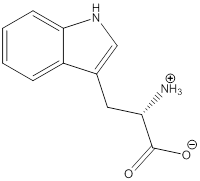
Zwitterion is one of the form in which the charge is separated and this is formed at a particular pH known as isoelectric point.
(c)
Interpretation:
Bond-line structure for the given set of amino acids in zwitterionic form need to be drawn.
Concept introduction:
Bond-line structure is the representation of organic structural formulas in a shorthand manner. In this case only the bond between carbon and other atoms are shown except hydrogen. It is understood that all the remaining valency to be filled by hydrogen. Apart from carbon atoms the other atoms are shown along with hydrogen.
Zwitterion is the one which has the positively and negatively charged groups separately. Zwitterion is found at the pH which is the isoelectric point.
To draw : To draw bond-line structure for L-glutamine
(c)
Answer to Problem 40PP
The bond-line structure for (c) and its zwitterion form is
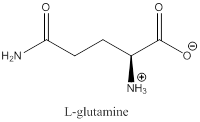
Explanation of Solution
Linear formula for L-glutamine
The linear formula for L-glutamine is
Draw bonds between the atoms except hydrogen
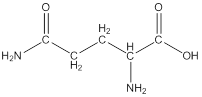
The structure from the linear formula is drawn as shown above. Considering the valency of carbon to be four, the structure is drawn.
Remove the carbons that are explicitly shown
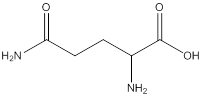
The carbon atoms are ignored from being explicitly shown as it is understood that the carbon and hydrogen atoms are not shown in bond-line structure.
Assign configuration
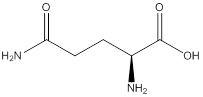
As in the name of the problem statement it is said L-glutamine, the chiral center must have “S” configuration and the same is shown in the above structure.
Zwitterion
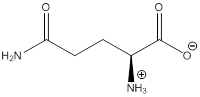
Zwitterion is one of the form in which the charge is separated and this is formed at a particular pH known as isoelectric point.
(d)
Interpretation:
Bond-line structure for the given set of amino acids in zwitterionic form need to be drawn.
Concept introduction:
Bond-line structure is the representation of organic structural formulas in a shorthand manner. In this case only the bond between carbon and other atoms are shown except hydrogen. It is understood that all the remaining valency to be filled by hydrogen. Apart from carbon atoms the other atoms are shown along with hydrogen.
Zwitterion is the one which has the positively and negatively charged groups separately. Zwitterion is found at the pH which is the isoelectric point.
To draw : To draw bond-line structure for L-proline
(d)
Answer to Problem 40PP
The bond-line structure for (d) and its zwitterion form is
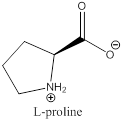
Explanation of Solution
Draw bonds between the atoms except hydrogen
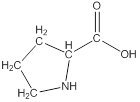
The structure from the linear formula is drawn as shown above. Considering the valency of carbon to be four, the structure is drawn.
Remove the carbons that are explicitly shown
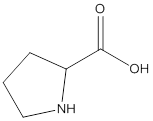
The carbon atoms are ignored from being explicitly shown as it is understood that the carbon and hydrogen atoms are not shown in bond-line structure.
Assign configuration
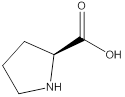
As in the name of the problem statement it is said L-proline, the chiral center must have “S” configuration and the same is shown in the above structure.
Zwitterion
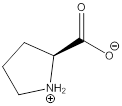
Zwitterion is one of the form in which the charge is separated and this is formed at a particular pH known as isoelectric point.
Want to see more full solutions like this?
Chapter 25 Solutions
ORGANIC CHEM LL +WILEYPLUSBB >IB<
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





