
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 25.SE, Problem 28VC
Interpretation Introduction
Concept introduction:
We redraw the pyranose cyclic structure of the given L aldohexose as follows:
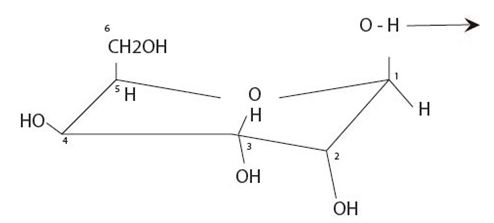
For the L sugar the CH2OH group is at the bottom of the ring.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
The 1H NMR spectrum of d-glucose in D2O exhibits two high-frequency doublets. What is responsible for these doublets?
Predict the products obtained when d-galactose reacts with each reagent.(a) Br2 and H2O (b) NaOH, H2O (c) CH3OH, H + (d) Ag(NH3) 2+ -OH(e) H2, Ni (f) excess Ac2O and pyridine (g) excess CH3 I, Ag2O (h) NaBH4(i) Br2, H2O, then H2O2 and Fe2(SO4)3 (j) (1) KCN/HCN; (2) H2, Pd/BaSO4; (3) H3O! (k) excess HIO42
The cyclic form of this sugar would be
A) alpha-anomer as the -CH2OH and the hemiacetal -OH are trans
B) beta-anomer as the -CH2OH and the hemiacetal -OH are cis
C) beta-anomer as the -CH2OH and the hemiacetal -OH are trans
D) alpha-anomer as the -CH2OH and the hemiacetal -OH are cis
Chapter 25 Solutions
Organic Chemistry
Ch. 25.1 - Prob. 1PCh. 25.2 - Prob. 2PCh. 25.2 - Prob. 3PCh. 25.2 - Prob. 4PCh. 25.2 - Prob. 5PCh. 25.3 - Prob. 6PCh. 25.3 - Prob. 7PCh. 25.4 - Prob. 8PCh. 25.4 - Prob. 9PCh. 25.4 - Prob. 10P
Ch. 25.5 - Prob. 11PCh. 25.5 - Prob. 12PCh. 25.5 - Prob. 13PCh. 25.5 - Prob. 14PCh. 25.5 - Prob. 15PCh. 25.6 - Prob. 16PCh. 25.6 - Prob. 17PCh. 25.6 - Prob. 18PCh. 25.6 - Prob. 19PCh. 25.6 - Prob. 20PCh. 25.6 - Prob. 21PCh. 25.6 - Prob. 22PCh. 25.6 - Prob. 23PCh. 25.7 - Prob. 24PCh. 25.8 - Show the product you would obtain from the...Ch. 25.SE - Prob. 26VCCh. 25.SE - Prob. 27VCCh. 25.SE - Prob. 28VCCh. 25.SE - Prob. 29VCCh. 25.SE - Prob. 30MPCh. 25.SE - Prob. 31MPCh. 25.SE - Glucosamine, one of the eight essential...Ch. 25.SE - D-Glicose reacts with acetone in the presence of...Ch. 25.SE - Prob. 34MPCh. 25.SE - Prob. 35MPCh. 25.SE - Prob. 36APCh. 25.SE - Prob. 37APCh. 25.SE - Prob. 38APCh. 25.SE - Prob. 39APCh. 25.SE - Prob. 40APCh. 25.SE - Assign R or S configuration to each chirality...Ch. 25.SE - Prob. 42APCh. 25.SE - Prob. 43APCh. 25.SE - Prob. 44APCh. 25.SE - Prob. 45APCh. 25.SE - Prob. 46APCh. 25.SE - Prob. 47APCh. 25.SE - Prob. 48APCh. 25.SE - Prob. 49APCh. 25.SE - Prob. 50APCh. 25.SE - Prob. 51APCh. 25.SE - Prob. 52APCh. 25.SE - Prob. 53APCh. 25.SE - Prob. 54APCh. 25.SE - Prob. 55APCh. 25.SE - Prob. 56APCh. 25.SE - Prob. 57APCh. 25.SE - Prob. 58APCh. 25.SE - Prob. 59APCh. 25.SE - Prob. 60APCh. 25.SE - Prob. 61APCh. 25.SE - Prob. 62APCh. 25.SE - Prob. 63APCh. 25.SE - D-Mannose reacts with acetone to give a...Ch. 25.SE - Prob. 65APCh. 25.SE - Prob. 66APCh. 25.SE - Prob. 67APCh. 25.SE - Prob. 68APCh. 25.SE - Prob. 69APCh. 25.SE - Prob. 70APCh. 25.SE - Prob. 71AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which configuration (R or S) does the bottom asymmetric carbon have for the d seriesof sugars? Which configuration for the l series?arrow_forwardd-Altrose is an aldohexose. Ruff degradation of d-altrose gives the same aldopentose asdoes degradation of d-allose, the C3 epimer of glucose. Give the structure of d-altrosearrow_forwardWhich of the d-aldopentoses will give optically active aldaric acids on oxidation with HNO3 ?(arrow_forward
- Is meclizine chiral? If so, how many of the possible stereoisomers are formed in this synthesis?arrow_forwardWhen d-fructose is dissolved in D2O and the solution is made basic, the d-fructose recovered from the solution has an average of 1.7 deuterium atomsattached to the C-1 carbon per molecule. Show the mechanism that accounts for the incorporation of these deuterium atoms into d-fructose.arrow_forwardAn aldopentose "O", is oxidized with HNO3 to a diacid "P" which is optically active. The compound "O", is also degraded to an aldotetrose "Q" which undergoes another oxidation to an optically inactive diacid "R". Assuming that "O" has the configuration D-(4R); what are the structures of "O" to "R"?arrow_forward
- D-Glicose reacts with acetone in the presence of acid to yield the nonreducing 1, 2: 5, 6-diisopropylidene-D-glucofuranose. Propose a mechanism.arrow_forwardOne of the later steps in glucose biosynthesis is the isomerization of fructose 6-phosphate to glucose 6-phosphate. Propose a mechanism, using acid or base catalysis as needed.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning