
(a)
Interpretation:
The structure of quinine in
Concept introduction:
Aliphatic
Answer to Problem 26.33AP
The structure of quinine in
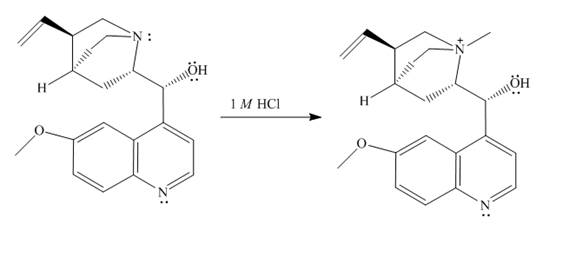
Explanation of Solution
When quinine reacts with
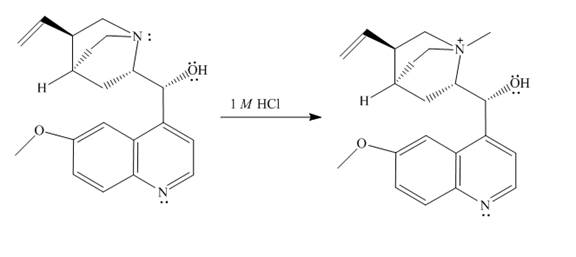
Figure 1
The structure of quinine in
(b)
Interpretation:
The structure of nicotine in
Concept introduction:
Aliphatic amines are more basic than aromatic amines. In aliphatic amines, the nitrogen atom is attached to a methyl group which is electron donating group; therefore, it increases the electron density on nitrogen by
Answer to Problem 26.33AP
The structure of nicotine in
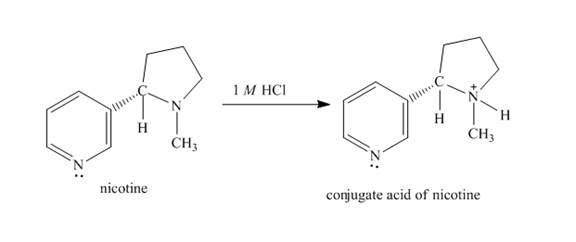
Explanation of Solution
When nicotine reacts with
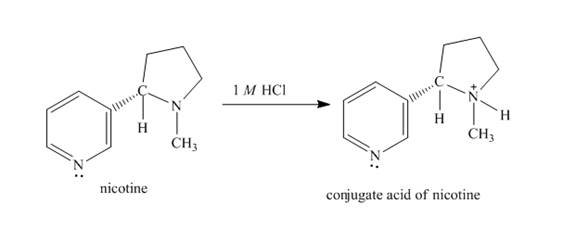
Figure 2
The structure of nicotine in
(c)
Interpretation:
The structure of tryptamine in
Concept introduction:
Aliphatic amines are more basic than aromatic amines. In aliphatic amines, the nitrogen atom is attached to a methyl group which is electron donating group; therefore, it increases the electron density on nitrogen by
Answer to Problem 26.33AP
The structure of tryptamine in
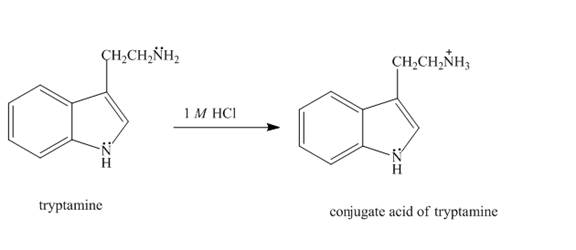
Explanation of Solution
The aliphatic amine present on the side chain of tryptamine gets protonated in

Figure 3
The structure of tryptamine in
(d)
Interpretation:
The structure of
Concept introduction:
Aliphatic amines are more basic than aromatic amines. In aliphatic amines, the nitrogen atom is attached to a methyl group which is electron donating group; therefore, it increases the electron density on nitrogen by
Answer to Problem 26.33AP
The structure of
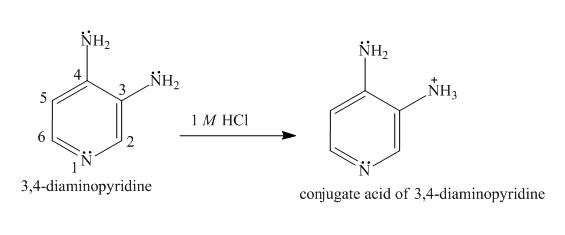
Explanation of Solution
In the structure of

Figure 4
The structure of
(e)
Interpretation:
The structure of
Concept introduction:
Aliphatic amines are more basic than aromatic amines. In aliphatic amines, the nitrogen atom is attached to a methyl group which is electron donating group; therefore, it increases the electron density on nitrogen by
Answer to Problem 26.33AP
The structure of
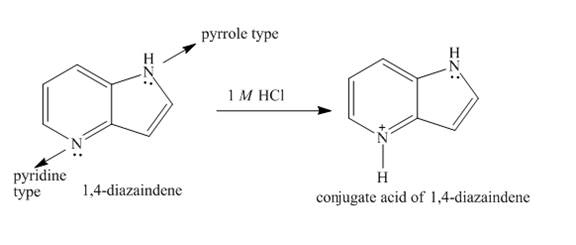
Explanation of Solution
In

Figure 5
The structure of
(f)
Interpretation:
The structure of
Concept introduction:
Aliphatic amines are more basic than aromatic amines. In aliphatic amines, the nitrogen atom is attached to a methyl group which is electron donating group; therefore, it increases the electron density on nitrogen by
Answer to Problem 26.33AP
The structure of
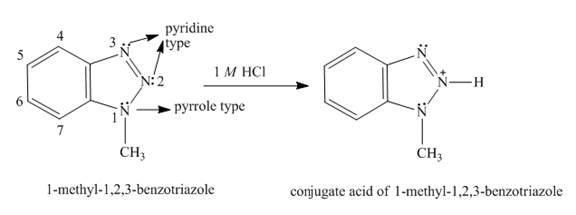
Explanation of Solution
In
The formation of conjugated acid of

Figure 6
The structure of
(g)
Interpretation:
The structure of imitanib in
Concept introduction:
Aliphatic amines are more basic than aromatic amines. In aliphatic amines, the nitrogen atom is attached to a methyl group which is electron donating group; therefore, it increases the electron density on nitrogen by
Answer to Problem 26.33AP
The structure of imitanib in
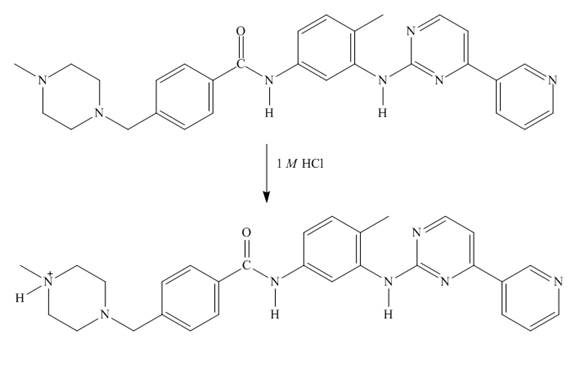
Explanation of Solution
In imitanib, the aliphatic amine will be protonated than aromatic amine since the basicity of aliphatic amines is more. The aliphatic amine present adjacent to the methyl group will be protonated as it is electron rich due to the
Figure 7
The structure of imitanib in
Want to see more full solutions like this?
Chapter 26 Solutions
Organic Chemistry 6e & Study Guide
- Give a clear handwritten answer with explanation needed...give the mechanism of given bleow reactionarrow_forwardExplain the differences in acidity between p-cyanobenzoic acid and m-cyanobenzoic acid. Illustrate your answer appropriately, showing the acid-base reaction that takes place with each of these and explaining in such terms as electronic (inductive or resonance) and/or steric effects.arrow_forwardExplain which amide (A, B or C) would be mainly found in the hexane layer, if there are all added to a water/hexane mixture?arrow_forward
- (b) Classify the following alcohols in order of increasing ease of acid-catalyzed dehydration.Justify your answers.arrow_forwardIn greater details and greater explanation, describe the differences you would see when conducting propyl amine with dimethyl amine. Be specific on what you would make sure you could distinguish the two amines.arrow_forwardGive the clear handwritten answer and give the mechanism of given bleow reactionsarrow_forward
- Give a clear handwritten answer with explanation and give the mechanismarrow_forwardGive the structure of azo dye that would be formed from the given amine and coupling component.arrow_forwardGive the clear handwritten answer with explanation...give the three possible products .....with major, minor productsarrow_forward
- Compound X was soluble in water and ether, and its aqueous solution turned litmus blue. It reacted with sodium to give a gas. The compound reacted with benzenesulfonyl chloride and base to give an insoluble product, which was unchanged with acidification. It reacted with nitrous acid to give a yellow solid. Compound A could bearrow_forwardGive a clear handwritten answer with textual explanation..give the mechanism of given bleow reactionsarrow_forwardGive a clear handwritten answer..give the mechanism with explanation needed!!!arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





