
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 26, Problem 26.38P
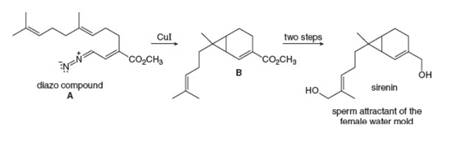
Although diazomethane
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Treatment of compound A (C8H17Br) with NaOCH2CH3 affords two constitutional isomers B and C. Ozonolysis of B affords CH2=O and (CH3CH2CH2)2C=O. Ozonolysis of C affords CH3CH2CH2COCH3 and CH3CH2CHO. What is the structure of A?
Muscalure is the sex attractant of the common housefly. Flies are lured to traps filled with bait thatcontain muscalure and an insecticide. Eating the bait is fatal. How could you synthesize muscalure using 1-bromopentane as one of the starting materials?
Although diazomethane (CH2N2) is often not a useful reagent forpreparing cyclopropanes, other diazo compounds give good yields ofmore complex cyclopropanes. Draw a stepwise mechanism for theconversion of diazo compound A to B, an intermediate in the synthesisof sirenin, the sperm attractant produced by the female gametes of thewater mold Allomyces.
Chapter 26 Solutions
Organic Chemistry
Ch. 26 - Prob. 26.1PCh. 26 - Prob. 26.2PCh. 26 - Prob. 26.3PCh. 26 - Prob. 26.4PCh. 26 - Prob. 26.5PCh. 26 - Prob. 26.6PCh. 26 - Prob. 26.7PCh. 26 - Problem 26.8
What starting materials are needed to...Ch. 26 - Prob. 26.9PCh. 26 - Problem 26.10
What reagents are needed to convert...
Ch. 26 - Problem 26.11
What product is formed when each...Ch. 26 - Prob. 26.12PCh. 26 - Problem 26.13
Draw the products formed when each...Ch. 26 - Problem 26.14
What products are formed when ...Ch. 26 - Problem 26.15
Draw the product formed from...Ch. 26 -
What product is formed by ring-closing metathesis...Ch. 26 - Problem 26.17
What starting material is needed to...Ch. 26 - 26.18 In addition to organic halides, alkyl...Ch. 26 - 26.19 What product is formed by ring-closing...Ch. 26 - 26.20 Draw the products formed in each...Ch. 26 - What organic halide is needed to convert lithium...Ch. 26 - 26.22 How can you convert ethynylcyclohexane to...Ch. 26 - 26.23 What compound is needed to convert styrene...Ch. 26 - 26.24 What steps are needed to convert to octane...Ch. 26 - Prob. 26.25PCh. 26 - Prob. 26.26PCh. 26 - 26.27 Draw the products (including stereoisomers)...Ch. 26 - 26.28 Treatment of cyclohexene with and forms...Ch. 26 - Prob. 26.29PCh. 26 - 26.30 What starting material is needed to prepare...Ch. 26 - Prob. 26.31PCh. 26 - Prob. 26.32PCh. 26 - When certain cycloalkenes are used in metathesis...Ch. 26 - 26.34 Draw the products formed in each reaction.
...Ch. 26 - Prob. 26.35PCh. 26 - Draw a stepwise mechanism for the following...Ch. 26 - Sulfur ylides, like the phosphorus ylides of...Ch. 26 - Although diazomethane is often not a useful...Ch. 26 - Prob. 26.39PCh. 26 - Prob. 26.40PCh. 26 - Prob. 26.41PCh. 26 - Prob. 26.42PCh. 26 - 26.43 Devise a synthesis of each compound using a...Ch. 26 - 26.44 Devise a synthesis of each compound from...Ch. 26 - 26.45 Devise a synthesis of each compound from...Ch. 26 - 26.46 Devise a synthesis of each substituted...Ch. 26 - Biaryls, compounds containing two aromatic rings...Ch. 26 - Prob. 26.48PCh. 26 - 26.49 Draw the product formed from the...Ch. 26 - Prob. 26.50PCh. 26 - 26.51 Devise a synthesis of each of the following...Ch. 26 - Prob. 26.52PCh. 26 - 26.53 The following conversion, carried out in the...Ch. 26 - Prob. 26.54PCh. 26 - 26.55 Dimethyl cyclopropanes can be prepared by...Ch. 26 - Prob. 26.56P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nicotinic acid, more commonly named niacin, is one of the B vitamins. Show how nicotinic acid can be converted to (a) ethyl nicotinate and then to (b) nicotinamide.arrow_forward-Ocimene is a pleasant-smelling hydrocarbon found in the leaves of certain herbs. It has the molecular formula C10H16 and a UV absorption maximum at 232 nm. On hydrogenation with a palladium catalyst, 2,6-dimethyloctane is obtained. Ozonolysis of -ocimene, followed by treatment with zinc and acetic acid, produces the following four fragments: (a) How many double bonds does -ocimene have? (b) Is -ocimene conjugated or nonconjugated? (c) Propose a structure for -ocimene. (d) Write the reactions, showing starting material and products.arrow_forwardA step in a synthesis of PGE1 (prostaglandin E1, alprostadil) is the reaction of a trisubstituted cyclohexene with bromine to form a bromolactone. Propose a mechanism for formation of this bromolactone and account for the observed stereochemistry of each substituent on the cyclohexane ring. Alprostadil is used as a temporary therapy for infants born with congenital heart defects that restrict pulmonary blood flow. It brings about dilation of the ductus arteriosus, which in turn increases blood flow in the lungs and blood oxygenation.arrow_forward
- Rank the compounds in each group in order of increasing reactivity in nucleophilic acyl substitution. CH3CH2CO2H, (CH3CH2CO)2O, CH3CH2CONHCH3arrow_forwardThe biosynthesis of lanosterol from squalene has intrigued chemists since its discovery. It is now possible, for example, to synthesize polycyclic compounds from acyclic or monocyclic precursors by reactions that form several C—C bonds in a single reaction mixture. a. Draw a stepwise mechanism for the following reaction.b. Show how X can be converted to 16,17-dehydroprogesterone.arrow_forwardTreatment of compound A (C8H17Br) with NaOCH2CH3 affords twoconstitutional isomers B and C. Ozonolysis of B affords CH2=O and(CH3CH2CH2)2C=O. Ozonolysis of C affords CH3CH2CH2COCH3 andCH3CH2CHO. What is the structure of A?arrow_forward
- 1. The ketone 2-heptanone has been identified as contributing to the odor of a number of dairy products, including condensed milk and cheddar cheese. Describe the synthesis of 2-heptanone from acetylene and any necessary organic and inorganic reagents. 2. Compound A has the molecular formula C14H25Br and was obtained by reaction of sodium acetylide (HC≡CNa) )with 1,12-dibromododecane. On treatment of compound A with sodium amide, it was converted to compound B (C14H24). Ozonolysis of compound B gave the diacid HO2C(CH2)12CO2H. Catalytic hydrogenation of compound B over Lindlar palladium gave compound C (C14H26), while hydrogenation over platinum gave compound D (C14H28). Sodium-ammonia reduction of compound B gave compound E (C14H26). Both C and E yielded O═CH(CH2)12CH═O on ozonolysis. Assign structures to compound A through E so as to be consistent with the observed transformations.arrow_forward2-bromo-2-methylbutane undergoes hydrolysis reaction with water, H2O toform compound W. Compound X and compound Y are produced when 2-bromo-2-methylbutane undergoes elimination reaction with alcoholic ofsodium hydroxide, NaOH. (ii) What is the type of reaction involved in the formation of compound W? (iii) Identify the major product of the elimination reaction between compound Xand compound Y based on Zaitsev’s rule.arrow_forwardWhen (ft)-6-bromo-2,6-dimethylnonane is dissolved in CH3OH, nucleophilic substitution yields an optically inactive solution. When the isomeric halide (fl)-2-bromo-2,5- dimethylnonane is dissolved in CH3OH under the same conditions, nucleophilic substitution forms an optically active solution. Draw the products formed in each reaction, and explain why the difference in optical activity is observed.arrow_forward
- Brevicomin, the aggregation pheromone of the western pine bark beetle, contains a bicyclic bridged ring system and is prepared by the acidcatalyzed cyclization of 6,7-dihydroxy-nonan-2-one. a. Suggest a structure for brevicomin. b. Devise a synthesis of 6,7-dihydroxynonan-2-one from 6-bromohexan-2- one. You may also use three-carbon alcohols and any required organic or inorganic reagents.arrow_forwardExplain briefly by illustrations the chemistry behind each answer 1. Preamble :A reaction flask contains a 2-bromopentane in an ethanolic solution of sodium ethoxide at room temperature and results in the formation of two olefinic products(1-pentene and 2-pentene) I) What reaction pathway is followed by the reaction above?A. E2 dehydrohalogenation B. E1 dehydrohalogenationC. SN1 dehydrohalogenationD. SN2 dehydrohalogenationE. A mixture of E1 and E2 pathways II) What is responsible for the formation of different products (major and minor).A. The different activated complex involved in the mechanism.B.Bimolecular Nucleophilic substitution reaction C.Bimolecular Elimination reaction D.The presence of sodium ethoxideE.The hybridisation nature of the secondary carbocation III) All the following is true about the reaction in question 1 except?A. The reaction follows Zaistev’s ruleB. Sodium ethoxide is the nucleophile in this reactionC. The more highly substituted alkene is the most stable…arrow_forwardFrom what you have learned about enols and the hydration of alkynes, predict what product is formed by the acid-catalyzed hydration of CH3CH2CH2C = COCH3. Draw a stepwise mechanism that illustrates how it is formed.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY