
Concept explainers
Devise a synthesis of each of the following compounds. Besides inorganic reagents, you may use hydrocarbons and halides having
a. c.
c. e.
e.
b.![]() d.
d.![]() f.
f.
(a)
Interpretation: The synthesis of the given compound is to be devised.
Concept introduction: Lithium aluminum hydride is a strong reducing agent. Treatment of carbonyl compounds with lithium aluminum hydride yields alcohols. It reduces ketone into secondary alcohol.
Answer to Problem 26.49P
The synthesis of the given compound is,
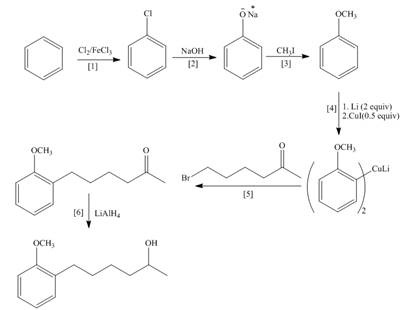
Explanation of Solution
The synthesis of given compound is follows:
Step-1 Chlorination of benzene takes place in the presence of ferric chloride and gives chlorobenzene.
Step-2 Chlorobenzene is treated with base and undergoes nucleophilic substitution reaction and gives phenol.
Step-3 Phenol is treated with methyl iodide and gives anisole.
Step-4 Anisole is treated with
Step-5 Organocuprate compound is treated with
Step-6 The substituted product is treated with
The synthesis of the given compound is shown below.
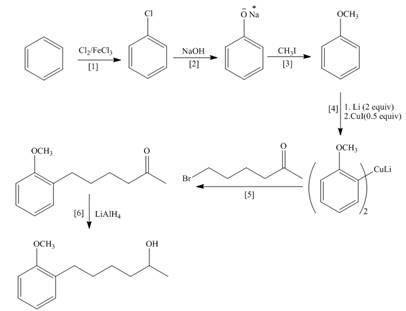
Figure 1
The synthesis of given compound is shown in Figure 1.
(b)
Interpretation: The synthesis of the given compound is to be devised.
Concept introduction: Gilman reagents are organometallic compounds which are a source of nucleophiles. These nucleophiles give
Answer to Problem 26.49P
The synthesis of the given compound is,
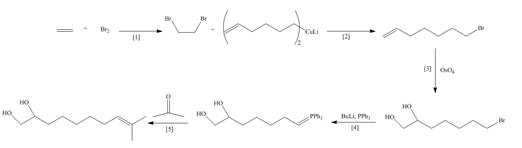
Explanation of Solution
The synthesis of the given compound is follows:
Step-1 Alkene is treated with
Step-2
Step-3The
Step-4 The compound obtained from step-4 is treated with
Step-5 The Wittig reagent is treated with acetone to give a desired product.
The synthesis of the given compound is shown below.

Figure 2
The synthesis of the given compound is shown in Figure 2.
(c)
Interpretation: The synthesis of the given compound is to be devised.
Concept introduction: Suzuki reaction is a type of substitution reaction. It involves the palladium coupling between organic halide with organoborane in the presence of base. It is a stereospecific reaction.
Answer to Problem 26.49P
The synthesis of the given compound is,
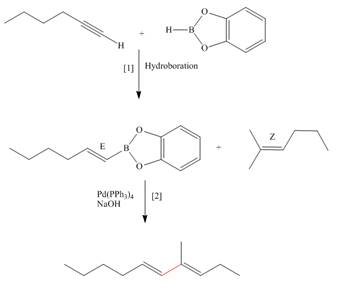
Explanation of Solution
The synthesis of the given compound is follows:
Step-1 Hydroboration of hex
Step-2 The
The synthesis of the given compound is shown below.
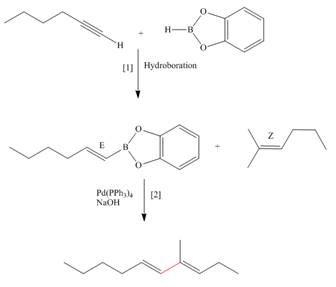
Figure 3
The synthesis of the given compound is shown in Figure 3.
(d)
Interpretation: The synthesis of the given compound is to be devised.
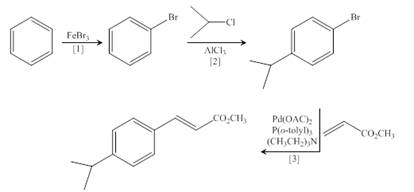
Concept introduction: Heck reaction is a type of substitution reaction. It involves the palladium coupling between aryl or vinyl halide with alkene which leads to the formation of trans alkene at less substituted carbon.
Answer to Problem 26.49P
The synthesis of the given compound is,
Explanation of Solution
The synthesis of the given compound is follows:
Step-1 Bromination of benzene takes place in the presence of ferric chloride and gives bromobenzene.
Step-2 Friedelcraft alkylation of bromobenzene takes place in the presence of isopropyl chloride.
Step-3The
The synthesis of the given compound is shown below.
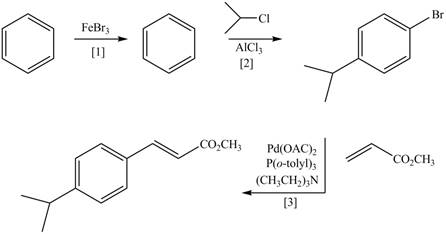
Figure 4
The synthesis of the given compound is shown in Figure 4.
(e)
Interpretation: The synthesis of the given compound is to be devised.
Concept introduction: Heck reaction is a type of substitution reaction. It involves the palladium coupling between aryl or vinyl halide with alkene which leads to the formation of trans alkene at less substituted carbon. The nonhalogenated cyclopropanes are synthesized by the treatment of an alkene with
Answer to Problem 26.49P
The synthesis of given compound is,
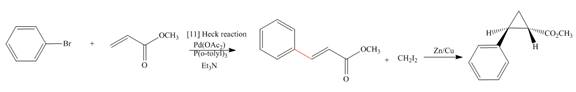
Explanation of Solution
The synthesis of given compound is as follows:
Step-1 Bromobenzene is treated with
Step-2 The compound formed in step-1 undergoes Simmons-Smith reaction and forms a desired product.
The synthesis of the given compound is shown below.
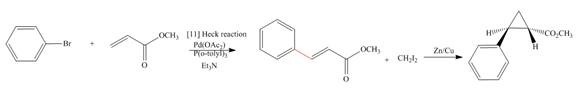
Figure 5
The synthesis of given compound is shown in Figure 5.
(f)
Interpretation: The synthesis of given compound is to be devised.
Concept introduction: Heck reaction is a type of substitution reaction. It involves the palladium coupling between aryl or vinyl halide with alkene which leads to the formation of trans alkene at less substituted carbon.
Answer to Problem 26.49P
The synthesis of given compounds is,
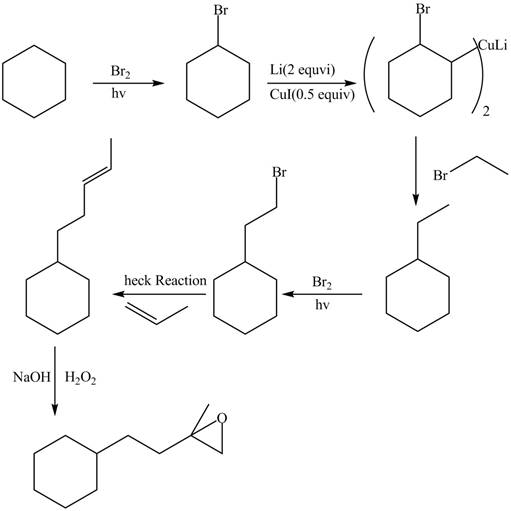
Explanation of Solution
The synthesis of given compounds is as follows:
Step-1 Cyclohexane is treated with
Step-2 The brominated compound then treated with
Step-3 The organocuprate compound then treated with organic bromide to form ethylcyclohexane.
Step-4 Then ethylcyclohexane undergoes heck reaction in the presence of 1-propene to form substituted alkene compound.
Step-5 The substituted alkene compound reacts with hydrogen peroxide in the sodium hydroxide to form the desired oxirane product.
The synthesis of the given compound is shown below.
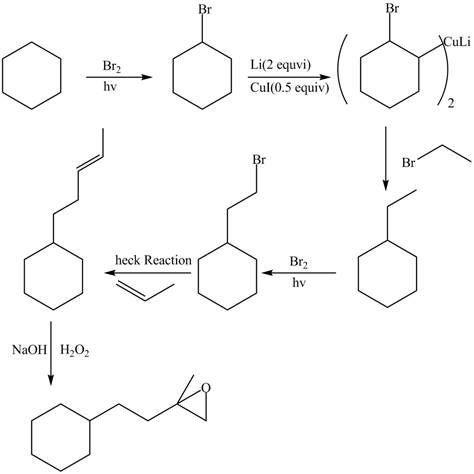
Figure 6
The synthesis of given compound is shown in Figure 6.
Want to see more full solutions like this?
Chapter 26 Solutions
Organic Chemistry -Study Guide / Solution Manual (Custom)
- Nicotinic acid, more commonly named niacin, is one of the B vitamins. Show how nicotinic acid can be converted to (a) ethyl nicotinate and then to (b) nicotinamide.arrow_forwardgive the reagents for parts a-parrow_forwardDEET is an active ingredient in many insect repellants, such as OFFTM Starting with meta-bromotoluene and using any other reagents of your choice, devise an efficient synthesis of DEET.arrow_forward
- Devise a synthesis of 1-phenyl-5-methylhexane [C6H5(CH2)4CH(CH3)2] from acetylene, alkyl halides, and any required inorganic reagents.arrow_forward17. Provide a synthesis of CH3CH2CH2 CH2 SH from any alkyl halide. Show the structure of all reactants and products.arrow_forwardWhat are the various functions of phenolics and Give seperately industrial uses of phenolics. Answer two pagesarrow_forward
- The name for Reaction 1 is _____ while Reaction 2 is called _____. Choices: A. Williamson Ether synthesis, B. Hydration, C. Epoxidation, D. Acidic cleavage The reagent/s for REACTION 1 is/are ______ Choices: A. m-chloroperoxybenzoic acid, B. H2O/H3PO4, C. H3O+, D. HI The reagent/s for REACTION 2 is/are ______ Choices: A. m-chloroperoxybenzoic acid, B. water in acidic medium, C. dilute acid, D. hydroiodic acidarrow_forwardDevise a synthesis of each of the following compounds. Besides inorganic reagents, you may use hydrocarbons and halides having ≤ 6 C′s, and CH2=CHCOOCH3 as starting materials. Each synthesis must use at least one of the carbon–carbon bond-forming reactions.arrow_forwardWhat is the major organic product obtained from the following reaction under low temperature conditions? A, B, C, or D?arrow_forward
- Devise a synthesis of each compound using 1-bromobutane(CH3CH2CH2CH2Br) as the only organic starting material. You may useany other inorganic reagents.arrow_forwardThe compound eutypine is an antibacterial agent isolated from the fungus Eutypa lata. This fungus results in a disease common to vineyards called eutyposis. Give a sequence of reactions that will take the following reactant and give eutypine when the other reactants used in the sequence are acetylene and acetone.arrow_forwardFill in the missing reagents below.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


