
Concept explainers
(a)
Interpretation: To determine whether oxaloacetate is associated with (1) transamination, (2) oxidative deamination, or (3) the urea cycle.
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
A biochemical reaction in which an
A urea cycle is a cyclic biochemical pathway that involves the production of urea using ammonium ions and aspartate molecules as nitrogen sources. The reactants in the formation of carbamoyl phosphate are ammonium ion, water, and carbon dioxide.
(a)
Answer to Problem 26.73EP
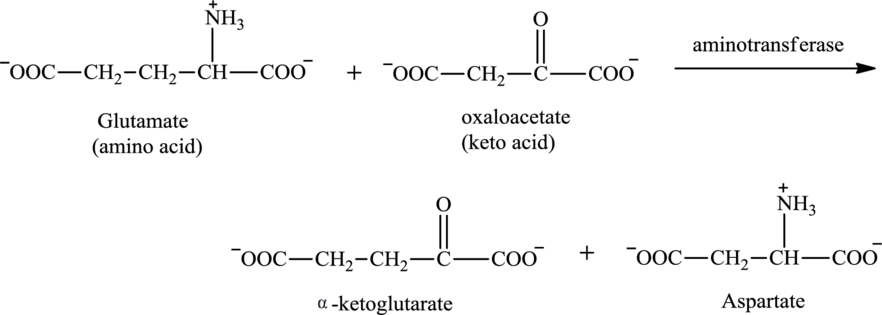
Oxaloacetate is associated with the transamination reaction.
Explanation of Solution
The structure of oxaloacetate is:
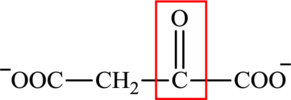
Oxaloacetate contains both a carbonyl group and a carboxyl functional group. Oxaloacetate is a keto acid. It could function as a reactant in the transamination reaction. It reacts with an amino acid to produce aspartate.
(b)
Interpretation: To determine whether arginine is associated with (1) transamination, (2) oxidative deamination, or (3) the urea cycle.
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
A biochemical reaction in which an
A urea cycle is a cyclic biochemical pathway that involves the production of urea using ammonium ions and aspartate molecules as nitrogen sources. The reactants in the formation of carbamoyl phosphate are ammonium ion, water, and carbon dioxide.
(b)
Answer to Problem 26.73EP
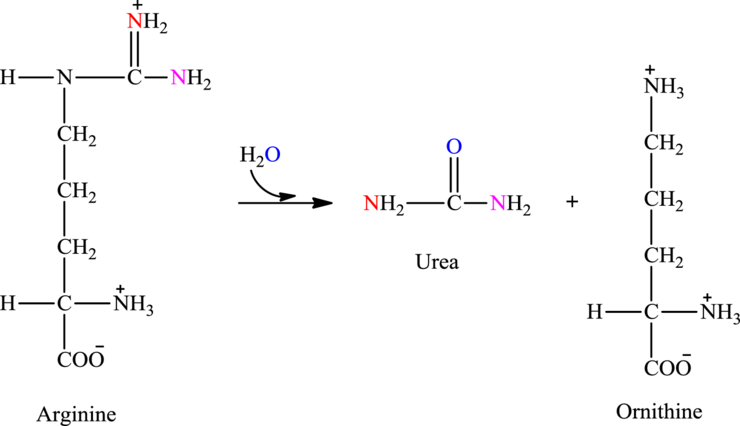
Arginine is associated with the urea cycle.
Explanation of Solution
In step 3 of the urea cycle, catalyst argininosuccinate lyase catalyzes the cleavage of argininosuccinate into arginine and fumarate. Arginine formed step 3 reacts with water and undergo hydrolysis in step 4.
Hydrolysis of arginine produce urea and also regenerates the starting fuel ornithine.
(c)
Interpretation: To determine whether
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
A biochemical reaction in which an
A urea cycle is a cyclic biochemical pathway that involves the production of urea using ammonium ions and aspartate molecules as nitrogen sources. The reactants in the formation of carbamoyl phosphate are ammonium ion, water, and carbon dioxide.
(c)
Answer to Problem 26.73EP
Water
Explanation of Solution
Water is one of the reactants in oxidative deamination reaction. For example, glutamate is an
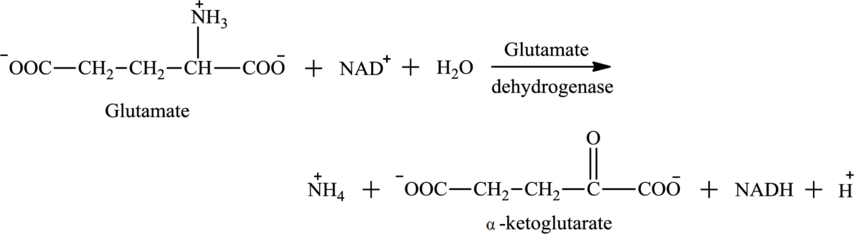
Water is also associated with the urea cycle. Ammonium ion produced from oxidative deamination reaction, carbon dioxide, water, and two ATP molecules reacts to form carbamoyl phosphate. Carbamoyl phosphate is fuel for the urea cycle. The
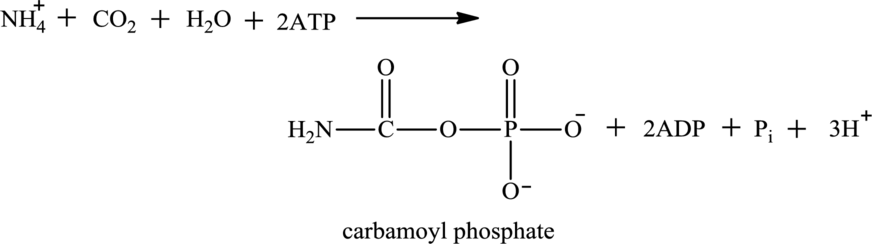
(d)
Interpretation: To determine whether ATP is associated with (1) transamination, (2) oxidative deamination, or (3) the urea cycle.
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
A biochemical reaction in which an
A urea cycle is a cyclic biochemical pathway that involves the production of urea using ammonium ions and aspartate molecules as nitrogen sources. The reactants in the formation of carbamoyl phosphate are ammonium ion, water, and carbon dioxide.
(d)
Answer to Problem 26.73EP
ATP is associated with the urea cycle.
Explanation of Solution
Adenosine triphosphate (ATP) is a molecule that is defined as the energy currency of life and provides energy to carry out the
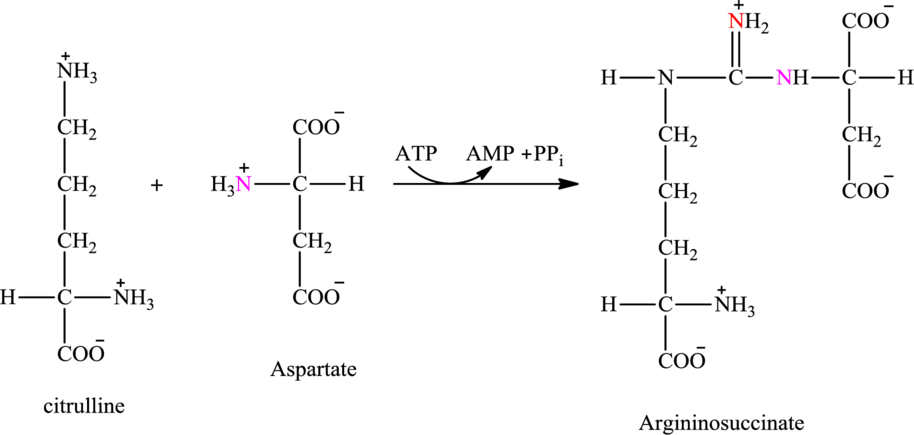
Step 2 of the urea cycle involves a condensation reaction of citrulline with aspartate to produce argininosuccinate. The reaction is carried out with the expenditure of ATP molecule.
Want to see more full solutions like this?
Chapter 26 Solutions
Bundle: General, Organic, And Biological Chemistry, Loose-leaf Version, 7th + Lms Integrated For Owlv2 With Mindtap Reader, 4 Terms (24 Months) ... Chemistry (powered By Owlv2), 4 Terms (2
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



