
Concept explainers
Convert each ball-and-stick model to a Fischer projection.
a.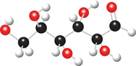 b.
b. 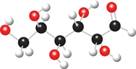
(a)
Interpretation: The given ball-and-stick model is to be converted into Fischer projection.
Concept introduction: In Fischer projection formula, the horizontal and vertical line represents the bonds that are present above and below the plane, respectively. The verticals bonds are represented as dashed wedge and horizontal bonds as dark wedge.
Answer to Problem 36P
The Fischer projection of given ball and stick model is shown below.
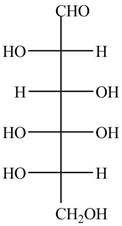
Figure 1
Explanation of Solution
The ball and stick model of given compound is,
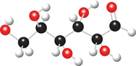
Figure 2
Black colored atoms have four bonds. So, these are the carbon atoms. The grey colored balls have one bond. So, these are the hydrogen atoms. The red colored atoms have two bonds. So, these are oxygen atoms. The molecular structure of given compound is,
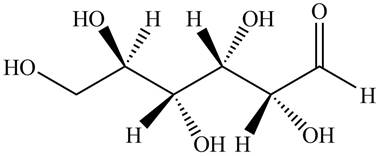
Figure 3
The conversion of given compound into Fisher projection is as follows:
The structure
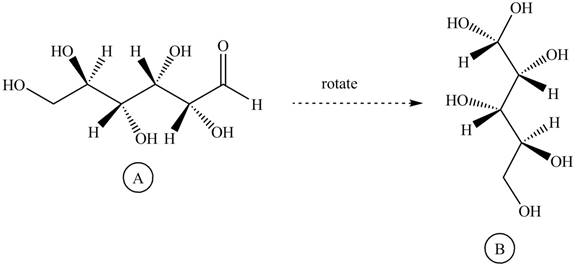
Figure 4
Rotate all the bonds around the carbon atom in such way that all staggered form converted into the eclipsed form in structure
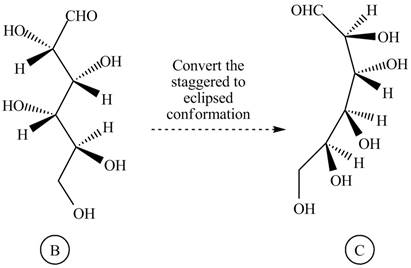
Figure 5
The structure
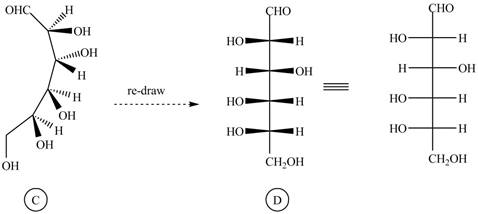
Figure 6
The Fischer projection of given ball and stick model is shown in Figure 1.
(b)
Interpretation: The products of given reaction are to be drawn.
Concept introduction: In Fischer projection formula, the horizontal and vertical line represents the bonds that are present above and below the plane, respectively. The verticals bonds are represented as dashed wedge and horizontal bonds as dark wedge.
Answer to Problem 36P
The Fischer projection of given ball and stick model is shown below.
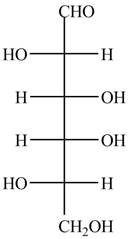
Figure 7
Explanation of Solution
The ball and stick model of given compound is,
Figure 8
Black colored atoms have four bonds. So, these are the carbon atoms. The grey colored balls have one bond. So, these are the hydrogen atoms. The red colored atoms have two bonds. So, these are oxygen atoms. The molecular structure of given compound is,
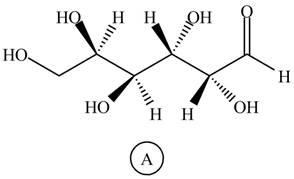
Figure 9
The conversion of given compound into Fisher projection is follow:
The structure
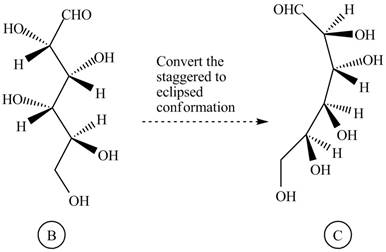
Figure 10
Rotate all the bonds around the carbon atom in such way that all staggered form converts into the eclipsed form in structure
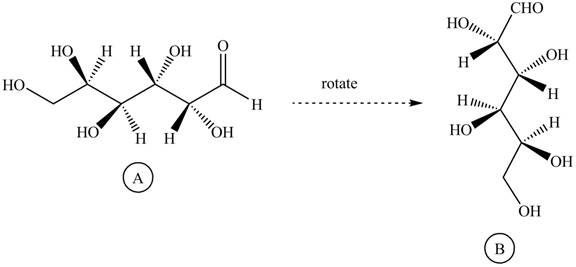
Figure 11
The structure
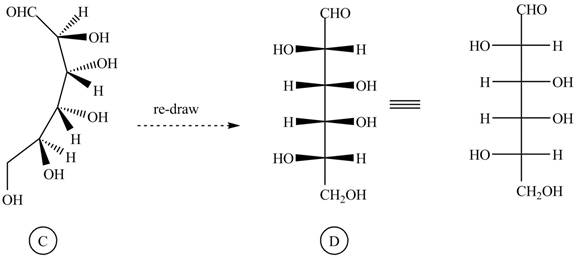
Figure 12
The Fischer projection of given ball and stick model is shown in Figure 7.
Want to see more full solutions like this?
Chapter 26 Solutions
ORGANIC CHEMISTRY (LL+SM+ACCESS)
- Convert each compound to a Fischer projection, and label each stereogenic center as R or S.arrow_forwardConsider the ball-and-stick model of A, and label B and C as either identical to A or an enantiomer of A.arrow_forwardRe-draw each Fischer projection formula using wedges and dashed wedgesfor the stereogenic center, and label the center as R or S.arrow_forward
- Celery ketone, like carvone, has two distinct aromas. In contrast to the S enantiomer's licorice scent, the R enantiomer has an earthy, celery-like aroma. Each enantiomer should be depicted and its odor assigned.arrow_forwardConvert each compound to a Fischer projection, and label eachstereogenic center as R or S.arrow_forwardDraw the michenisamarrow_forward
- Draw a Fischer projection for each compound. Remember that the cross represents an asymmetric carbon atom, and the carbon chain should be along the vertical, with the IUPAC numbering from top to bottom.(R)-glyceraldehyde,arrow_forwardDraw a Fischer projection for each compound. Remember that the cross represents an asymmetric carbon atom, and the carbon chain should be along the vertical, with the IUPAC numbering from top to bottom.(R)-butan-2-olarrow_forwardClassify each compound as IDENTICAL to A or an ENANTIOMER to A.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





