
Concept explainers
(a)
Interpretation:
The peptide
Concept introduction:
Amino acids are classified as acidic, basic and neutral according to the functional group they possesses. Acidic amino acids are those which have one more than one carboxylic acid group in its side chain and basic amino acids are those which have one or more amino group present in their side chain. Isoelectric point of the amino acids is the pH of the dilute aqueous solution of the amino acid at which the total charge on all molecules of amino acid is zero.
Answer to Problem 27.10P
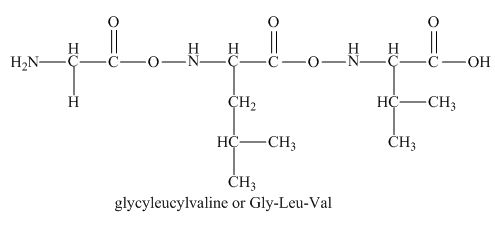
The peptide
There will be no charge on the peptide,
Explanation of Solution
Peptides are classified as acidic, basic, or neutral according to the amino acids they contain. If they contain unbalanced basic amino acid then they are considered as basic peptide and if they contain unbalanced acidic amino acid then they are considered as basic peptides. If the basic and acidic amino acids are balanced or not at all present in the peptide then it is considered as neutral peptide.
The given peptide

Figure 1
This tripeptide contains one amino group and one carboxylic group. So, this amino acid is neutral in nature. The
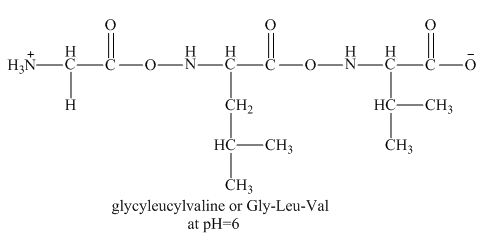
Figure 2
Therefore, the net charge on the peptide,
The peptide
(b)
Interpretation:
The peptide,
Concept introduction:
Amino acids are classified as acidic, basic and neutral according to the functional group they possesses. Acidic amino acids are those which have one more than one carboxylic acid group in its side chain and basic amino acids are those which have one or more amino group present in their side chain. Isoelectric point of the amino acids is the pH of the dilute aqueous solution of the amino acid at which the total charge on all molecules of amino acid is zero.
Answer to Problem 27.10P
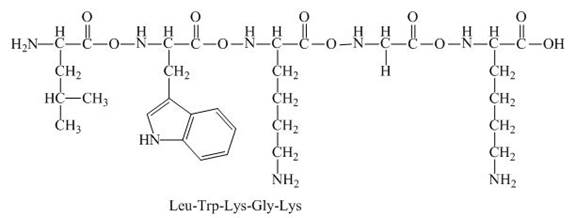
The peptide,
The net charge on the peptide,
Explanation of Solution
Peptides are classified as acidic, basic, or neutral according to the amino acids they contain. If they contain unbalanced basic amino acid then they are considered as basic peptide and if they contain unbalanced acidic amino acid then they are considered as basic peptides. If the basic and acidic amino acids are balanced or not at all present in the peptide then it is considered as neutral peptide.
The given peptide,

Figure 3
This given peptide contains three amino group in its chain and one carboxylic group. So, this amino acid is basic in nature. All the amine group of the given peptide having value of
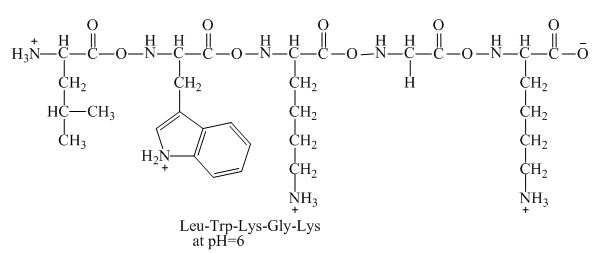
Figure 4
Therefore, the net charge of
The peptide,
(c)
Interpretation:
The peptide,
Concept introduction:
Amino acids are classified as acidic, basic and neutral according to the functional group they possesses. Acidic amino acids are those which have one more than one carboxylic acid group in its side chain and basic amino acids are those which have one or more amino group present in their side chain. Isoelectric point of the amino acids is the pH of the dilute aqueous solution of the amino acid at which the total charge on all molecules of amino acid is zero.
Answer to Problem 27.10P
The peptide
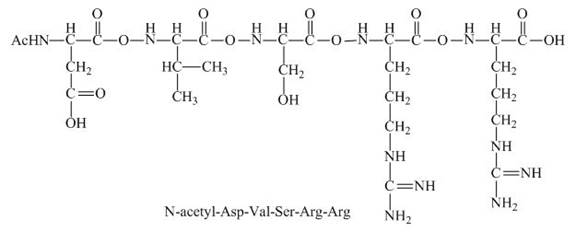
There will be no charge on the peptide,
Explanation of Solution
Peptides are classified as acidic, basic, or neutral according to the amino acids they contain. If they contain unbalanced basic amino acid then they are considered as basic peptide and if they contain unbalanced acidic amino acid then they are considered as basic peptides. If the basic and acidic amino acids are balanced or not at all present in the peptide then it is considered as neutral peptide.
The given peptide

Figure 5
This given peptide contains two amino groups in the side chain and two carboxylic groups. So, this amino acid is neutral in nature. All the amine groups of this peptide having
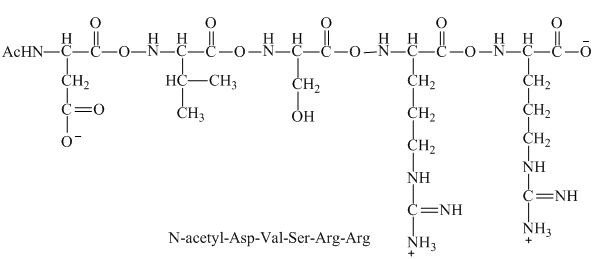
Figure 6
Therefore, the net charge on the peptide,
The peptide
(d)
Interpretation:
The peptide
Concept introduction:
Amino acids are classified as acidic, basic and neutral according to the functional group they possesses. Acidic amino acids are those which have one more than one carboxylic acid group in its side chain and basic amino acids are those which have one or more amino group present in their side chain. Isoelectric point of the amino acids is the pH of the dilute aqueous solution of the amino acid at which the total charge on all molecules of amino acid is zero.
Answer to Problem 27.10P
The peptide
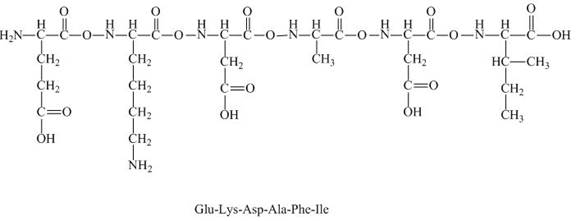
The charge present on the peptide,
Explanation of Solution
Peptides are classified as acidic, basic, or neutral according to the amino acids they contain. If they contain unbalanced basic amino acid then they are considered as basic peptide and if they contain unbalanced acidic amino acid then they are considered as basic peptides. If the basic and acidic amino acids are balanced or not at all present in the peptide then it is considered as neutral peptide.
The given peptide

Figure 7
This given peptide contains two amino groups in the side chain and four carboxylic groups. So, this amino acid is acidic in nature. All the amine groups of this peptide having
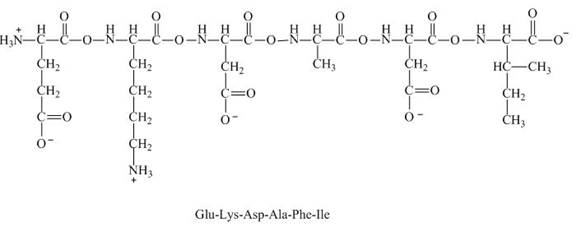
Figure 8
Therefore, the net charge on the peptide,
The peptide
Want to see more full solutions like this?
Chapter 27 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- 22-35 Why is histidine considered a basic amino acid when the pKa of its side chain is 6.0?arrow_forwardOn complete hydrolysis, a polypeptide gives two alanine, one leucine, one methionine, one phenylalanine, and one valine residue. Partial hydrolysis gives the following fragments: Ala-Phe, Leu-Met, Val-Ala, Phe-Leu. It is known that the first amino acid in the sequence is valine and the last one is methionine. What is the complete sequence of amino acids?arrow_forward22-71 Which amino acid side chain is most frequently involved in denaturation by reduction?arrow_forward
- If you were to design a small peptide with a large net negative charge at physiological pH, which amino acid residues should predominate?arrow_forwardConsider the peptide Trp-Arg-Glu-Cys-Gly-Tyr. For the drawings requested below, please show them in zig-zag style, from amino to carboxy terminus, with correct stereochemistry please highlight the part has been change Draw the predominant form at pH = 2 Draw the predominant form at pH = 5 Draw the predominant form at pH = 7 Draw the predominant form at pH = 12arrow_forwardWhich of the following peptides have a net positive chargeat pH 7? (a) Gly-Ser-Lys, (b) Pro-Leu-Ile, (c) Phe-Tyr-Asp.arrow_forward
- Draw the structural formulas of the following peptides, andshow which bond would be cleaved by chymotrypsin.a. ala-phe-alab. tyr-ala-tyrarrow_forward1. A tetradecapeptide (14 amino acid residues) gives the following peptide fragments on partial hydrolysis.(i) From this information, deduce the primary structure of this polypeptide. Fragments are grouped according to size. Pentapeptide fragments Tetrapeptide fragments Phe-Val-Asn-Gln-His His-Leu-Cys-Gly-Ser Gly-Ser-His-Leu-Val Gln-His-Leu-Cys His-Leu-Val-Glu Leu-Val-Glu-Alaarrow_forwardCalculate the isoelectric pH of this peptide: His-Tyrarrow_forward
- Which of the following peptides have a net positive charge at pH 7? (a) Gly-Ser-Lysarrow_forwardDraw the structure of the following short peptide at pH 7.0. You don't need to show the stereochemistry. Label the peptide bonds, alpha carbons, the C-terminus, and the N-terminus. What is the net charge of this peptide at pH 5.0? Ala-Asn-Glu-Val-Pro-Glyarrow_forwardWhich one is Second prominent form of arginine at pH 6.0 in the picture below?arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning




