
Concept explainers
(a)
Interpretation:
The
Concept introduction:
In mass spectroscopy, compounds can be identified on the basis of the mass of the compound. When the compound breaks into fragment then they can be distinguished from the other compounds. This technique is also used to differentiate the isotopes of compounds. In amino acids, three types of fragments are observed in low energy collisions are a, b and y ions. It is known as tandem mass spectrometry.
Answer to Problem 27.27P
The
Where N is asparagine, F is phenylalanine, E is glutamic acid, S is serine, G is glycine, K is lysine amino acid.
Explanation of Solution
In amino acids, b-type fragments appear due to an amino group or in other words charge is being carried by N-terminal. That is why it is also known as the N-terminus amino acid fragment. The b-type fragment is shown below.
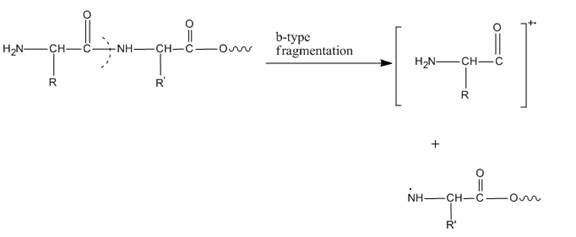
Figure 1
The given peptide is
The
(b)
Interpretation:
The
Concept introduction:
In mass spectroscopy, compounds can be identified on the basis of the mass of the compound. When the compound breaks into fragment then they can be distinguished from the other compounds. This technique is also used to differentiate the isotopes of compounds. In amino acids, three types of fragments are observed in low energy collisions are a, b and y ions. It is known as tandem mass spectrometry.
Answer to Problem 27.27P
The
Where N is asparagine, F is phenylalanine, E is glutamic acid, S is serine, G is glycine, K is lysine amino acid.
Explanation of Solution
In amino acids, y-type fragments appear due to a carboxyl group or in other words charge is being carried by C-terminal. That is why it is also known as the C-terminus amino acid fragment. The y-type fragment is shown below.
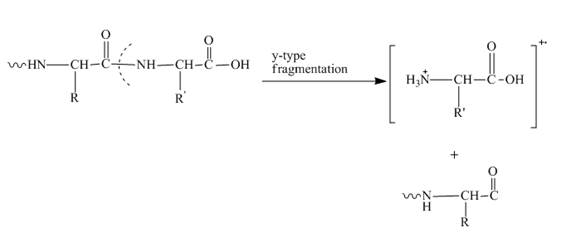
Figure 2
The given peptide is
The
Want to see more full solutions like this?
Chapter 27 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- Determine the buffer capacity of Alanine and Histidine at pH 2.0 and 6.0 respectively and discuss the results.arrow_forwardExplain the order of elution (with a buffer of pH 4) of the following pairs of amino acids through a column packed with Dowex 50a. aspartate before serine c. valine before leucineb. serine before alanine d. tyrosine before phenylalaninearrow_forwardThree peptides were obtained from a trypsin digestion of two different polypeptides. In each case, indicate the possible sequences from the given data and tell what further experiment should be carried out in order to determine the primary structure of the polypeptide. a. polypeptide I: 1. Val-Gly-Asp-Lys 2. Leu-Glu-Pro-Ala-Arg 3. Ala-Leu-Gly-Asp b. polypeptide II: 1. Val-Leu-Gly-Glu 2. Ala-Glu-Pro-Arg 3. Ala-Met-Gly-Lysarrow_forward
- Chemistry Find peptide RKCSPDD charge at 1, 7, and 14 pharrow_forwardUsing the information below, determine the amino acid sequence of the peptide, and explain how your structure is consistent with each piece of information. Complete hydrolysis by 6 M HCl at 110°C followed by amino acid analysis indicated the presence of Gly, Leu, Phe, and Tyr in a 2:1:1:1 molar ratio. Treatment of the peptide with1-fluoro-2,4-dinitrobenzene followed by complete hydrolysis and chromatography indicated the presence of the 2,4-dinitrophenyl derivative of tyrosine. No free tyrosine could be found. Complete digestion of he peptide with pepsin (which cleaves on the amino side of aromatic residues) followed by chromatography yielded a dipeptide containing Phe and Leu and a tripeptide containing Tyr and Gly in a 1:2 ratio.arrow_forwardA peptide has the following amino acid composition: 2 Met, 2 Phe, 2 Glu, 1 Arg, 1 Lys, 1 Val, 1 Leu, 1 Gly, 1 Ser Reaction of the intact peptide with dansyl chloride followed by acid hydrolysis creates a derivative of Met. A specific cleavage of the intact peptide produces fragments with the following sequences: Fragment A: Glu-Gly-Lys-Phe Fragment B: Met-Ser-Leu-Arg Fragment C: Met-Val-Glu-Phe What information do this result give about the sequence of the peptide? Explain how you arrived on your answer. a) The sequence is: Met-Val-Glu-Phe-Glu-Gly-Lys-Phe-Met-Ser-Leu-Arg b) The sequence is: Met-Ser-Leu-Arg-Met-Val-Glu-Phe-Glu-Gly-Lys-Phe c) The sequence is: Met-Val-Glu-Phe-Met-Ser-Leu-Arg-Glu-Gly-Lys-Phe d) The sequence is: Met-Ser-Leu-Arg-Glu-Gly-Lys-Phe-Met Val-Glu-Phearrow_forward
- 22-35 Why is histidine considered a basic amino acid when the pKa of its side chain is 6.0?arrow_forward22-61 Polyglutamic acid (a polypeptide chain made only of glutamic acid residues) has an a-helix conformation below pH 6.0 and a random-coil conformation above pH 6.0. What is the reason for this conformational change?arrow_forwardAn amino acid mixture of phenylalanine, glycine and glutamic acid is to be separated bypaper chromatography. The solvent is less polar than water. Which of these amino acids will have the highest Rf value and which the lowest? Explain. Treatment of a new protein with dansyl chloride reveals two (2) dansyl-labelled derivatives of amino acids, alanine and methionine. What can you deduce about the structure of the protein from these results? Name the amino acids that contribute atoms to both purine and pyrimidine rings.arrow_forward
- Calculate the percentage ionization (i.e. [base]/[acid]x100) in these amino acid side chains at the pH values specified (show your work for one of these amino acid chains): - Aspartate (pKa=4.1) at pH 5.4 - Arginine (pKa=12.5) at pH 11.8 - Histidine (pKa=6.0) at pH 8.0 - Tyrosine (pKa=10.9) at pH 8.0arrow_forwardCalculate the isoelectric pH of this peptide: His-Tyrarrow_forwardHis-Met-Asp-Tyr-Phe-Ser Calculate an approximate pI (isoelectric point) for this peptide.arrow_forward

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning


