
(a)
Interpretation: The starting materials that are needed to synthesize the given compound by a thermal
Concept introduction: A
Answer to Problem 27.40P
The starting materials that are needed to synthesize the given compound by a thermal
Explanation of Solution
The given product is shown below.
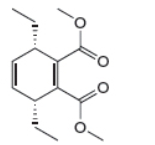
Figure 1
Retro synthesis can be carried out to identify the diene and dienophile for the given product.
The reaction that shows the disconnection approach of the given product is shown below.
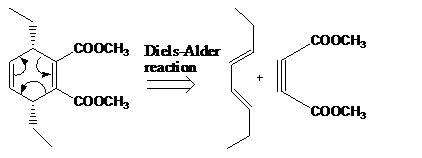
Figure 2
In the given product, the ring is opened due to the rearrangement of
The starting materials that are needed to synthesize the given compound by a thermal
(b)
Interpretation: The starting materials that are needed to synthesize the given compound by a thermal
Concept introduction: A chemical reaction that involves
Answer to Problem 27.40P
The starting materials that are needed to synthesize the given compound by a thermal
Explanation of Solution
The given product is shown below.
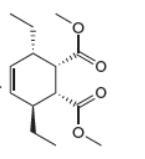
Figure 3
Retro synthesis can be carried out to identify the diene and dienophile for the given product.
The reaction that shows the disconnection approach of the given product is shown below.
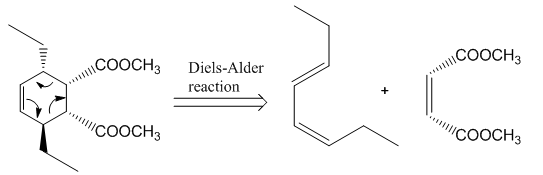
Figure 4
In the given product, the ring is opened due to the rearrangement of
Thus, the starting materials that are needed to synthesize the given compound by a thermal
The starting materials that are needed to synthesize the given compound by a thermal
(c)
Interpretation: The starting materials that are needed to synthesize the given compound by a thermal
Concept introduction: A chemical reaction that involves
Answer to Problem 27.40P
The starting materials that are needed to synthesize the given compound by a thermal
Explanation of Solution
The given product is shown below.
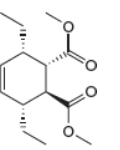
Figure 5
Retro synthesis can be carried out to identify the diene and dienophile for the given product.
The reaction that shows the disconnection approach of the given product is shown below.
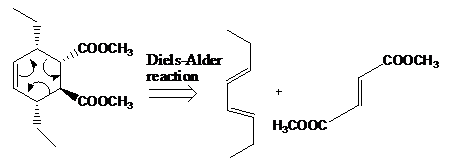
Figure 6
In the given product, the ring is opened due to the rearrangement of
Thus, the starting materials that are needed to synthesize the given compound by a thermal
The starting materials that are needed to synthesize the given compound by a thermal
Want to see more full solutions like this?
Chapter 27 Solutions
Connect Access Card For Organic Chemistry
- How can X be prepared from a constitutional isomer by a series of [2 + 2] cycloaddition reactions? Interest in molecules that contain several cyclobutane rings fused together has been fueled by the discovery of pentacycloanammoxic acid methyl ester, a lipid isolated from the membrane of organelles in the bacterium Candidatus Brocadia anammoxidans. The role of this unusual natural product is as yet unknown.arrow_forwardWhen buta-1,3-diene (CH2 = CH – CH = CH2) is treated with HBr, two constitutional isomers are formed, CH3CHBrCH = CH2 and BrCH2CH = CHCH3. Draw a stepwise mechanism that accounts for the formation of both products.arrow_forwardWhich isomer reacts more rapidly in an E2 reaction: cis-1-bromo-4-tert-butylcyclohexane or trans-1bromo-4-tert-butylcyclohexane? Explain your answer.arrow_forward
- What order of reagents should be used to synthesize the following product from methylcyclopentane?(A) Br2(B) Br2, hv(C) KOH, heatarrow_forwardHow many alkenes yield 2,3−dimethylbutane on catalytic hydrogenation?arrow_forwardWhat cyclic product is formed when each decatetraene undergoes thermal electrocyclic ring closure?arrow_forward
- What type of sigmatropic rearrangement is illustrated in each reaction?arrow_forwardWhat product(s) are formed in the following reaction. Indicate the proper stereochemistry of the product(s).arrow_forwardWhat type of cycloaddition occurs in Reaction [1]? Draw the product of asimilar process in Reaction [2]. Would you predict that these reactionsoccur under thermal or photochemical conditions?arrow_forward
- Consider a reaction where cis-but-2-ene is treated with OsO4 followed by NaHSO3/H2O. Draw the structure of one product that is formed in the reaction, including correct stereochemistry.arrow_forwardWhat is the major product formed from reacting cyclohexene in aqueous Br2, followed by treatment with potassium tert-butoxide?arrow_forwardHow can X be prepared from a constitutional isomer by a series of [2 + 2] cycloaddition reactions? Interest in molecules that contain several cyclobutane rings fused together has been fueled by the discovery of pentacycloanammoxic acid methyl ester, a lipid isolated from the membrane of organelles in the bacterium Candidatus Brocadia anammoxidans. Therole of this unusual natural product is as yet unknown.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





