
(a)
Interpretation: The product of the
Concept introduction: In the sigmatropic rearrangement, the rearrangement of pi bond and the breaking of sigma bond take place. This results in the generation of new sigma bond in the product. In this type of rearrangement, the number of pi bonds remains constant in the reactant as well as in the product.
Curved arrows aid in determining the movement and flow of electrons in the reaction. The electrons that take part in the
Answer to Problem 27.45P
The product of the
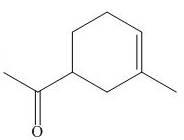
Explanation of Solution
The product of the
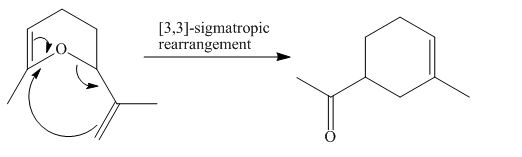 [a1]
[a1]
Figure 1
The above diagram indicates that in the product, both the
Thus, the name of product is
The product of the
(b)
Interpretation: The product of the
Concept introduction: In the sigmatropic rearrangement, the rearrangement of pi bond and the breaking of sigma bond take place. This results in the generation of new sigma bond in the product. In this type of rearrangement, the number of pi bonds remains constant in the reactant as well as in the product.
Curved arrows aid in determining the movement and flow of electrons in the reaction. The electrons that take part in the chemical reactions are shown by the curved arrows.
Answer to Problem 27.45P
The product of the
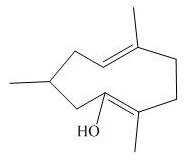
Explanation of Solution
The product of the
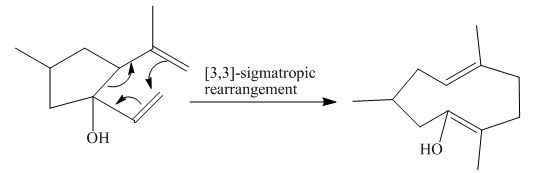
Figure 2
The above diagram indicates that in the product, both the
Thus, the name of product is
The product of the
(c)
Interpretation: The product of the
Concept introduction: In the sigmatropic rearrangement, the rearrangement of pi bond and the breaking of sigma bond take place. This results in the generation of new sigma bond in the product. In this type of rearrangement, the number of pi bonds remains constant in the reactant as well as in the product.
Curved arrows aid in determining the movement and flow of electrons in the reaction. The electrons that take part in the chemical reactions are shown by the curved arrows.
Answer to Problem 27.45P
The product of the
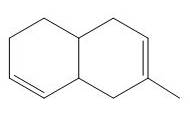
Explanation of Solution
The product of the
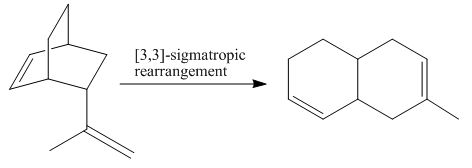
Figure 3
The above diagram indicates that in the product, both the
Thus, the name of product is
The product of the
Want to see more full solutions like this?
Chapter 27 Solutions
Organic Chemistry
- Draw all products formed by treatment of each dibromide (A and B) with one equivalent of NaNH2arrow_forwardAddition of HCl to alkene X forms two alkyl halides Y and Z. (A) Label Y and Z as a kinetic or thermodynamic product and explain why. (B) Explain why the addition of HCl occurs at the exocyclic C=C, rather than the other C=Carrow_forwardDraw a stepwise mechanism and all stereoisomers formed following reaction.arrow_forward
- Draw a stepwise mechanism for the attached reaction, which involves two Friedel–Crafts reactions. B was an intermediate in the synthesis of the antidepressant sertralinearrow_forwardpls draw a stepwise mechanism for the reaction thanks!arrow_forwardDraw the products of each reaction, and include the stereochemistry at any stereogenic center in the products (d.)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





