
Organic Chemistry Study Guide and Solutions Manual, Books a la Carte Edition
8th Edition
ISBN: 9780134473178
Author: Bruice, Paula Yurkanis
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 27, Problem 44P
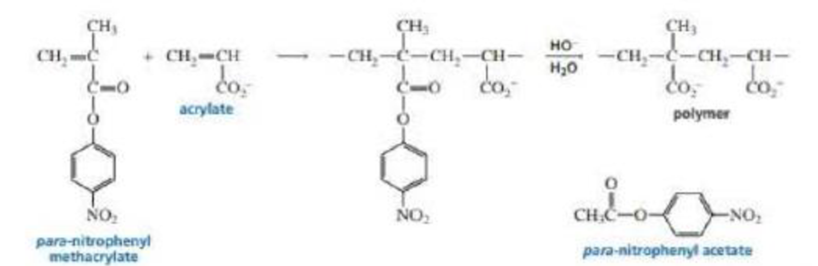
The
- a. Propose a mechanism for the formation of the copolymer.
- b. Explain why hydrolysis of the copolymer to form the polymer occurs much more rapidly than hydrolysis of para-nitrophenyl acetate.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Poly(vinyl alcohol), a hydrophilic polymer used in aqueous adhesives, is made by polymerizing vinyl acetate and then hydrolyzing the ester linkages. We have seen that basic hydrolysis destroys the Dacron polymer. Poly(vinyl acetate) is converted to poly(vinyl alcohol) by a basic hydrolysis of the ester groups. Why doesn’t the hydrolysis destroy the poly(vinyl alcohol)polymer?
Poly(vinyl alcohol), a hydrophilic polymer used in aqueous adhesives, is made by polymerizing vinyl acetate and then hydrolyzing the ester linkages. Why is poly(vinyl alcohol) made by this circuitous route? Why not just polymerize vinyl alcohol?
One common type of cation exchange resin is prepared by polymerization of a mixture containing styrene and 1,4-divinylbenzene . The polymer is then treated with concentrated sulfuric acid to sulfonate a majority of the aromatic rings in the polymer.
Q.) Show the product of sulfonation of each benzene ring.
Chapter 27 Solutions
Organic Chemistry Study Guide and Solutions Manual, Books a la Carte Edition
Ch. 27.3 - Prob. 1PCh. 27.3 - Prob. 2PCh. 27.3 - Prob. 3PCh. 27.3 - Prob. 4PCh. 27.3 - Prob. 5PCh. 27.3 - Prob. 6PCh. 27.4 - Prob. 7PCh. 27.5 - Rank the following groups of monomers from most...Ch. 27.5 - Why does methyl methacrylate not undergo cationic...Ch. 27.6 - Prob. 10P
Ch. 27.6 - Explain why, when propylene oxide undergoes...Ch. 27.6 - Which monomer and which type of initiator can you...Ch. 27.6 - Prob. 13PCh. 27.8 - Draw a short segment of gutta-percha.Ch. 27.8 - Prob. 15PCh. 27.11 - Prob. 16PCh. 27.11 - Write an equation that explains what happens if a...Ch. 27.11 - What happens to polyester slacks if aqueous NaOH...Ch. 27.11 - a. Propose a mechanism for the formation of the...Ch. 27.11 - Explain why, when a small amount of glycerol is...Ch. 27.12 - Propose a mechanism for the formation of melmac.Ch. 27.12 - Prob. 22PCh. 27.13 - Prob. 23PCh. 27 - Draw short segments of the polymers obtained from...Ch. 27 - Prob. 25PCh. 27 - Prob. 26PCh. 27 - Draw the structure of the monomer or monomers used...Ch. 27 - Prob. 28PCh. 27 - Draw short segments of the polymers obtained from...Ch. 27 - Quiana is a synthetic fabric that feels very much...Ch. 27 - Prob. 31PCh. 27 - Prob. 32PCh. 27 - Prob. 33PCh. 27 - Poly(vinyl alcohol) is a polymer used to make...Ch. 27 - Five different repeating units are found in the...Ch. 27 - Prob. 37PCh. 27 - A particularly strong and rigid polyester used for...Ch. 27 - Prob. 39PCh. 27 - Which Monomer gives a greater yield of polymer,...Ch. 27 - Prob. 41PCh. 27 - Prob. 42PCh. 27 - Why do vinyl raincoats become brittle as they get...Ch. 27 - The polymer shown below is synthesized by...Ch. 27 - Prob. 45PCh. 27 - How can head-to-head poly(vinyl bromide) be...Ch. 27 - Delrin (polyoxymethylene) is a tough...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Show the structure of the polymer that results from heating the following diepoxide and diamine:arrow_forwarda) Draw the structure of the prepolymer A formed from 1,4 dihydroxybenzene and excess epichlorohydrin. (b) Draw the structure of the cross-linked polymer B formed when A is treated with H2NCH2CH2CH2NH2 as the hardening agent.arrow_forward(a) Draw the structure of the prepolymer A formed from 1,4-dihydroxybenzene and excess epichlorohydrin. (b) Draw the structure of the cross-linked polymer B formed when A is treated with H2NCH2CH2CH2NH2 as the hardening agent.arrow_forward
- Why does Poly (DL-lactic acid) degrade more rapidly than Poly(L-lactic acid)?arrow_forward(a) Hard contact lenses, which first became popular in the 1960s, were made by polymerizing methyl methacrylate [CH2=C(CH3)CO2CH3] to form poly(methyl methacrylate) (PMMA). Draw the structure of PMMA. (b) More-comfortable softer contact lenses introduced in the 1970s were made by polymerizing hydroxyethyl methacrylate [CH2=C(CH3)CO2CH2CH2OH] to form poly(hydroxyethyl methacrylate) (poly-HEMA). Draw the structure of poly-HEMA. Because neither polymer allows oxygen from the air to pass through to the retina, newer contact lenses that are both comfortable and oxygen-permeable have now been developed.arrow_forwardShow the first three steps (as far as the tetramer) in the BF3@catalyzed polymerization of propylene to form polypropylene.arrow_forward
- Commercial polyisobutylene (PIB or butyl rubber) is synthesized via cationic polymerization. Explain why it cannot be made through free radical or anionic polymerizationarrow_forwardWhen styrene (vinylbenzene) is commercially polymerized, about 1–3% of 1,4-divinylbenzene is often added to thestyrene. The incorporation of some divinylbenzene gives a polymer with more strength and better resistance to organicsolvents. Explain how a very small amount of divinylbenzene has a marked effect on the properties of the polymerarrow_forwardRadical polymerization of styrene gives a linear polymer. Radical polymerization of a mixture of styrene and 1,4-divinylbenzene gives a cross-linked network polymer of the type shown in Figure 29.1. Show by drawing structural formulas how incorporation of a few percent of 1,4-divinylbenzene in the polymerization mixture gives a cross-linked polymer.arrow_forward
- Show the intermediate that would result if the growing chain added to the other end of the styrene double bond. Explain why the final polymer has phenyl groups substituted on every other carbon atom rather than randomly distributedarrow_forwardGive a single benefit for each polymerisation from carrying them out under a nitrogen atmosphere? (d) Of the two polymers produced (A and B) one had Mn = 12,000 and a polydispersity of 1.1 and one a Mn = 11, 200 and a polydispersity of 1.7. Identify which set of molecular weight parameters corresponds to which polystyrene sample.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you

 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
CBSE Class 12 Chemistry || Polymers || Full Chapter || By Shiksha House; Author: Best for NEET;https://www.youtube.com/watch?v=OxdJlS0xZ0Y;License: Standard YouTube License, CC-BY