
Concept explainers
(a)
Interpretation:
One alternative tautomeric structure for Adenine has to be written.
Concept Introduction:
The isomers which differ in the position of the protons and electrons and the carbon skeleton is same are said to be Tautomers and the mechanism is known as Tautomerism. The common example for Tautomerism is keto-enol tautomerization. The keto group is converted to enol (alcohol) group by the intramolecular exchange of protons.
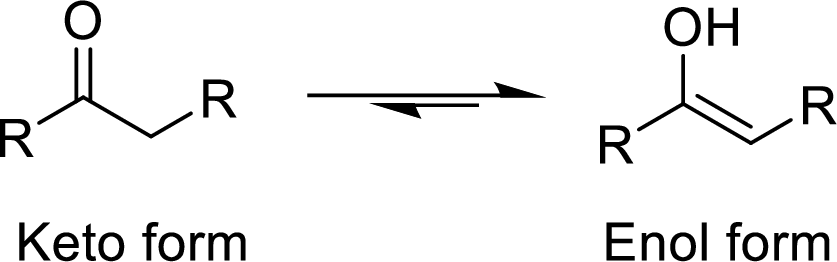
(b)
Interpretation:
Will the tautomeric structure of Adenine base pairs with Thymine or any other base has to be given.
Concept Introduction:
In the DNA double helix, the bases are paired with each other on the adjacent strands to hold the double helix. The bases present in DNA are Adenine, Guanine, Cytosine and Thymine. The pairing occurs between the Adenine and Thymine (A-T) and Guanine and Cytosine (G-C).
Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
Organic Chemistry
- On complete hydrolysis, a polypeptide gives two alanine, one leucine, one methionine, one phenylalanine, and one valine residue. Partial hydrolysis gives the following fragments: Ala-Phe, Leu-Met, Val-Ala, Phe-Leu. It is known that the first amino acid in the sequence is valine and the last one is methionine. What is the complete sequence of amino acids?arrow_forwardA. Lysine is considered a basic amino acid containing a guanidino group. If all ionizable protons from lysine were deprotonated, what will be the overall charge for the lysine? B. Which of the following is true regarding amino acids? -Leucine and isoleucine have the same molecular mass. -Glycine is a chiral molecule. -Proline is considered to be an ⍺-amino acid. -Lysine (short for L) is a basic amino acid.arrow_forwardA 1.00-mg sample of a pure protein yielded on hydrolysis 0.0165 mg of leucine and 0.0248 mg of isoleucine. What is the minimum possible molar mass of the protein? (MMleucine=MMisoleucine=131g/mol)arrow_forward
- Give the sequence of the following tetrapeptide:arrow_forwardSuppose that 28% of the nucleotides in a DNA molecule are deoxythymidine 5'-monophosphate, and that during DNA replication the percentage amounts of available nucleotide bases art 22% A, 22% C, 28% G. and 28% T. Which base would be depleted first in the replication process?arrow_forwardConstruct the primary structure of the polypeptide MethionylLeucylGlycylLeucylValine in trans configuration and write its nomenclaturearrow_forward
- What observations led Linus Pauling and his colleaguesto hypothesize that the peptide bond exists as a resonancehybrid?arrow_forwardWhen RNA is hydrolysed, there is no relationship among the quantities ofdifferent bases obtained. What does this fact suggest about the structureof RNA.arrow_forwardIf you were to design a small peptide with a large net negative charge at physiological pH, which amino acid residues should predominate?arrow_forward
- Identify an amino acid that complies with the following characteristic: (Write the COMPLETE name) A side chain that reacts with HCl via acid-base reactionarrow_forwardWhich of these molecules is not a natural amino acid?arrow_forwardWatson and Crick determined that the DNA molecule has two helical strands. Each strand is made up of , which consist of a base, a deoxyribose, and a phosphate group linked together. What word most correctly fills in the blank in the preceding statement? A.amino acids b.monosaccharides c.fatty acids d.nucleotidesarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





