
Concept explainers
(a)
Interpretation:
In conjugated triene,
Concept introduction:
The molecular orbital is a combination of two atomic orbitals. It is used to represent the regions in a molecule where the electron is likely to be present in an orbital. It represents the wave-like nature of an electron in a molecule. It may be symmetric or antisymmetric. It may be bonding, antibonding or non-bonding. It may be HOMO or LUMO.
Answer to Problem 28.2P
In
Explanation of Solution
The structure of
The electron configuration of carbon atom is
Figure 1
The atomic p orbital of carbon atom overlapped together to form molecular orbital. A total of six molecular orbitals are formed due to six carbon atomic orbital overlapping in
(b)
Interpretation:
Each molecular orbital is to be classified as symmetric and antisymmetric.
Concept introduction:
The molecular orbital is a combination of two atomic orbitals. It is used to represent the regions in a molecule where the electron is likely to be present in an orbital. It represents the wave-like nature of an electron in a molecule. It may be symmetric or antisymmetric. It may be bonding, antibonding or non-bonding. It may be HOMO or LUMO.
Answer to Problem 28.2P
The molecular orbitals
Explanation of Solution
In molecular orbital theory, the MO is said to be symmetric or anti-symmetric is depend on the relative phase of the two terminal carbons. In symmetric MO, the peaks reflect across the reference plane into the peaks and troughs reflect into troughs. On the other hand, in antisymmetric MO, the peaks reflect into troughs and vice versa. According to the general principle, the even number molecular orbitals are antisymmetric and odd number molecular orbitals are symmetric. Therefore, the molecular orbitals
The odd number of molecular orbitals is symmetric molecular orbitals that are
(c)
Interpretation:
Bonding and antibonding molecular orbitals are to be identified.
Concept introduction:
The molecular orbital is a combination of two atomic orbitals. It is used to represent the regions in a molecule where the electron is likely to be present in an orbital. It represents the wave-like nature of an electron in a molecule. It may be symmetric or antisymmetric. It may be bonding, antibonding or non-bonding. It may be HOMO or LUMO.
Answer to Problem 28.2P
The molecular orbital
Explanation of Solution
In
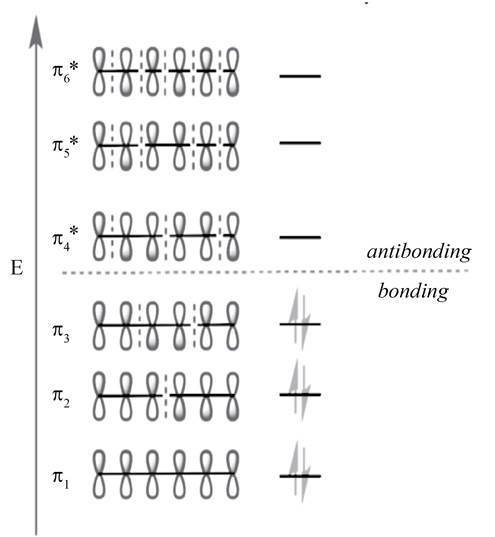
Figure 2
The molecular orbital with lower energy are bonding MO
(d)
Interpretation:
Among the molecular orbitals, the frontier molecular orbital is to be identified.
Concept introduction:
The molecular orbital is a combination of two atomic orbitals. It is used to represent the regions in a molecule where the electron is likely to be present in an orbital. It represents the wave-like nature of an electron in a molecule. It may be symmetric or antisymmetric. It may be bonding, antibonding or non-bonding. It may be HOMO or LUMO.
Answer to Problem 28.2P
The molecular orbital
Explanation of Solution
The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) is known as frontier molecular orbitals. In
The molecular orbital
(e)
Interpretation:
Whether the phase at terminal carbons in HOMO orbitals is the same or different is to be stated.
Concept introduction:
The molecular orbital is a combination of two atomic orbitals. It is used to represent the regions in a molecule where the electron is likely to be present in an orbital. It represents the wave-like nature of an electron in a molecule. It may be symmetric or antisymmetric. It may be bonding, antibonding or non-bonding. It may be HOMO or LUMO.
Answer to Problem 28.2P
The HOMO orbital that is
Explanation of Solution
According to the general principle, the even number molecular orbitals are antisymmetric or its phase terminal carbons are different and odd number molecular orbitals are symmetric or its phase terminal carbons are same. The HOMO orbital is
The HOMO orbital is
(f)
Interpretation:
Whether the phase at terminal carbons in LUMO orbitals is the same or different is to be stated.
Concept introduction:
The molecular orbital is a combination of two atomic orbitals. It is used to represent the regions in a molecule where the electron is likely to be present in an orbital. It represents the wave-like nature of an electron in a molecule. It may be symmetric or antisymmetric. It may be bonding, antibonding or non-bonding. It may be HOMO or LUMO.
Answer to Problem 28.2P
The LUMO orbital that is
Explanation of Solution
According to the general principle, the even number molecular orbitals are antisymmetric or its phase terminal carbons are different and odd number molecular orbitals are symmetric or its phase terminal carbons are same. The LUMO orbital is
The LUMO orbital is
Want to see more full solutions like this?
Chapter 28 Solutions
Loose-leaf Version For Organic Chemistry
- Explain and give examples of the stability order of carbocations within the group and among different groupsarrow_forwardDescribe with a suitable example why the characterisation of chiral compounds is important to the pharmaceutical industry and indicate why separation of the enantiomers can prove challenging. Explain how capillary electophoresis can be used in the analysis of these compounds.arrow_forwardAnswer the following question for 1,3,5- hexatriene, the conjugated triene containing six carbons. Which MOs are the frontier molecular orbitals?arrow_forward
- Answer the following questions for 1,3,5- hexatriene, the conjugated triene containing six carbons. Which MOs are the frontier molecular orbitals?arrow_forwardhow can we distinguish between Fe2 and Fe3 by using AAS method?arrow_forward1. Briefly discuss the important application of tris(triphenylphospine) chlorodium in organic chemistry.illustrating your answer with suitable examplearrow_forward
- A chemist is attempting to synthesize a complex natural product with a highly strained cyclohexene ring system. Which type of reactants would be most suitable for achieving this goal, and why? Provide a detailed explanation of the choice of reactants and the expected outcome in terms of the Diels-Alder reaction.arrow_forwardThe molecular formula of unknown compound B is C10H16.Quantitative brominationof a 0.473-g sample of compound Brequired 48.22mL of a 0.216MBr2/CCl4to produce a color change. How many rings and pi bonds does compound Bcontain?Include any explanations/calculations to justify your answers.arrow_forwardGive a clear explanation handwritten in detailed of both subparts pleasearrow_forward
- Using the Frost Circle method to outline the molecular orbitals of cyclobutadiene. and identify whether it is aromatic, antiaromatic or non-aromatic. Explain. (Note: Show the outline of MO of cyclobutadiene using the Frost circle method)arrow_forwardAs the extent of electron delocalization into the ring increases, the geometry at nitrogen flattens. p-Nitroaniline, for example, is planar. Write a resonance contributor for p-nitroaniline that shows how the nitro group increases electron delocalization.arrow_forwardJust do compound Carrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
