
Concept explainers
(a)
Interpretation: The product formed by the treatment of
Concept introduction: Monosaccharides are the small units of simple sugars. The hydroxyl groups of monosaccharides are converted into the ether groups in presence of base and
Answer to Problem 28.49P
The product formed by the treatment of
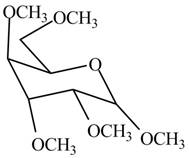
Figure 1
Explanation of Solution
The conversion of hydroxyl groups of
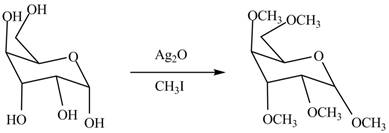
Figure 2
The product formed by the treatment of
(b)
Interpretation: The product formed by the treatment of
Concept introduction: Monosaccharides are the small units of simple sugars The hydroxyl groups of monosaccharides are converted into the ether groups in presence of base and alkyl halide.
Answer to Problem 28.49P
The products formed by the treatment of
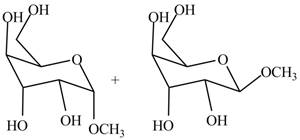
Figure 3
Explanation of Solution
The two anomers are formed by the treatment of monosaccharides with methanol in the presence of hydrochloric acid. One product with methoxy group on the equatorial position and other product with methoxy group on the axial position. The corresponding chemical reaction is shown below.
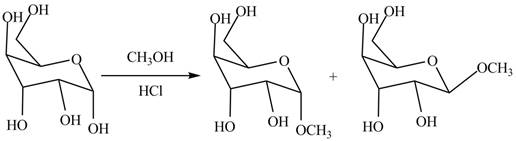
Figure 4
The products formed by the treatment of
(c)
Interpretation: The product formed by the treatment of
Concept introduction: Monosaccharides are the small units of simple sugars. The hydroxyl groups of monosaccharides are converted into the ether groups in presence of base and alkyl halide.
Answer to Problem 28.49P
The product formed by the treatment of
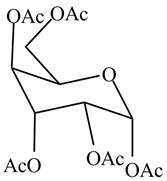
Figure 5
Explanation of Solution
In the presence of acetic anhydride and pyridine, the conversion of alcoholic groups in
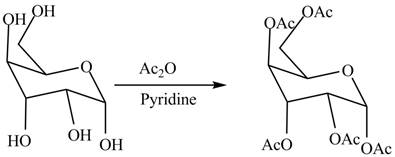
Figure 6
The product formed by the treatment of
(d)
Interpretation: The product formed by the treatment of
Concept introduction: Monosaccharides are the small units of simple sugars. The hydroxyl groups of monosaccharides are converted into the ether groups in presence of base and alkyl halide.
Answer to Problem 28.49P
The products formed by the treatment of
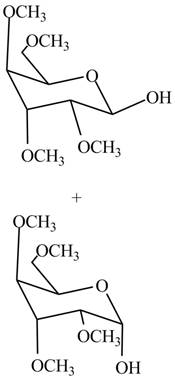
Figure 7
Explanation of Solution
The first step is the conversion of hydroxyl groups of gulose into the ether groups in presence of base and methyl iodide as shown in Figure 2.
The product
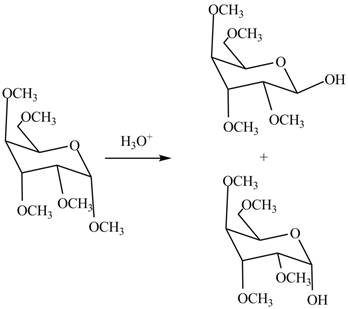
Figure 8
The products formed by the treatment of
(e)
Interpretation: The product formed by the treatment of
Concept introduction: Monosaccharides are the small units of simple sugars The hydroxyl groups of monosaccharides are converted into the ester groups in presence of base and acetic anhydride or acetyl chloride.
Answer to Problem 28.49P
The products formed by the treatment of
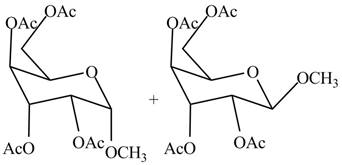
Figure 9
Explanation of Solution
The conversion of alcoholic groups of product
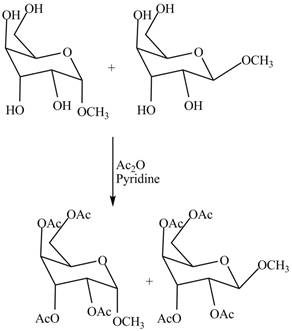
Figure 10
The products formed by the treatment of
(f)
Interpretation: The product formed by the treatment of
Concept introduction: Monosaccharides are the small units of simple sugars. The hydroxyl groups of monosaccharides are converted into the ether groups in presence of base and alkyl halide.
Answer to Problem 28.49P
The products formed by the treatment of
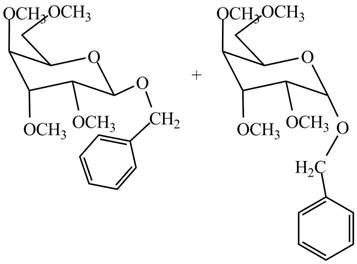
Figure 11
Explanation of Solution
The conversion of hydroxyl groups of product
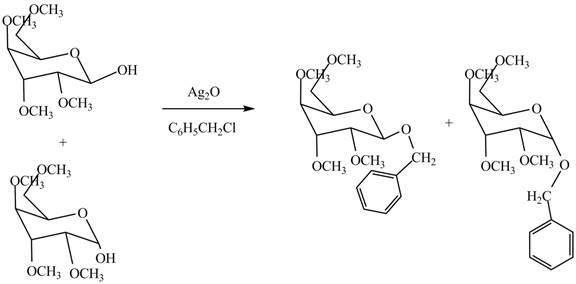
Figure 12
The products formed by the treatment of
Want to see more full solutions like this?
Chapter 28 Solutions
Package: Organic Chemistry with Connect 2-year Access Card
- Draw the product formed when (CH3)2CHOH is treated with each reagent. a.SOCl2, pyridine b. TsCl, pyridine c.H2SO4 d.HBr e.PBr3, then NaCN f.POCl3, pyridinearrow_forwardDraw the product formed when (CH3)2CHOH is treated with each reagent. a. SOCl2, pyridine b. TsCl, pyridine c. H2SO4 d. HBr e. PBr3, then NaCN f. POCl3, pyridinearrow_forwardDraw the product formed when (CH3)2CHOH is treated with each reagent (a, b and c)arrow_forward
- Draw the products formed when (CH3)2C=CH2 is treated with following reagent. [1] BH3; [2] H2O2, HO−arrow_forwardDraw the product formed when (CH3)2CHOH is treated with following reagent. POCl3, pyridinearrow_forwardDraw the product formed when (CH3)2CHOH is treated with following reagent. H2SO4arrow_forward
- Draw the products formed when hex-1-yne is treated with each reagent. a. HCl (2 equiv) b. HBr (2 equiv) c. Cl2 (2 equiv) d.H2O + H2SO4 + HgSO4 e. [1] R2BH; [2] H2O2, HO− f. NaH g. [1] −NH2; [2] CH3CH2Brarrow_forwardDraw the product formed when C6H5N2 +Cl− reacts with each compound.arrow_forwardDraw the product formed when C6H5N2+Cl− reacts with each compound.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





