
(a)
Interpretation:
The resonance contributors for given species has to be drawn.
Concept Introduction:
Localized electrons:
If the negative charge formed by losing a proton resides only on one atom, they are termed as localized electrons. For example, if an alcohol loses a proton, the electrons remaining will be resides on its single oxygen atom.
Delocalized electrons:
If the negative charge formed by losing a proton resides on more than two atoms, they are termed as delocalized electrons. For example, if carboxylic acid loses a proton, the electrons remaining will be resides on both oxygen atoms and thus reduces the electron density of the atom making the conjugate base more stable.
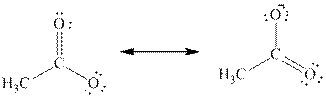
The above two structures are known as resonance contributors and the actual structure which is called the resonance hybrid will be the composite of the two resonance contributors.
(b)
Interpretation:
The resonance contributors for given species has to be drawn.
Concept Introduction:
Localized electrons:
If the negative charge formed by losing a proton resides only on one atom, they are termed as localized electrons. For example, if an alcohol loses a proton, the electrons remaining will be resides on its single oxygen atom.
Delocalized electrons:
If the negative charge formed by losing a proton resides on more than two atoms, they are termed as delocalized electrons. For example, if carboxylic acid loses a proton, the electrons remaining will be resides on both oxygen atoms and thus reduces the electron density of the atom making the conjugate base more stable.
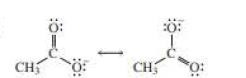
The above two structures are known as resonance contributors and the actual structure which is called the resonance hybrid will be the composite of the two resonance contributors.
Trending nowThis is a popular solution!

Chapter 2 Solutions
Organic Chemistry
- Procyclidine is a drug that has been used to treat the uncontrolled body movements associated with Parkinson’s disease. Draw three different methods to prepare procyclidine using a Grignard reagent.arrow_forwardConsider the following: Why is the oxygen atom selectively protonated? A) Oxygen is less electronegative than nitrogen. B) The six-membered ring becomes aromatic when protonated. C) Oxygen is a better electrophile. D) Better stabilization by resonance.arrow_forwardwhat are the reagents to convert A to Barrow_forward
- What steps are needed to convert A to B?arrow_forwardBromoetherification, the addition of the elements of Br and OR to a double bond, is a common method for constructing rings containing oxygen atoms. This reaction has been used in the synthesis of the polyether antibiotic monensin (Problem 18.34). Draw a stepwise mechanism for the following intramolecular bromoetherification reaction.arrow_forwardsusceptible to nucleophilic attack a to b c only a only a and carrow_forward
- can you identify A and Barrow_forwardBromoetherication, the addition of the elements of Br and OR to a double bond, is a common method for constructing rings containing oxygen atoms. This reaction has been used in the synthesis of the polyether antibiotic monensin (Problem 21.37). Draw a stepwise mechanism for the following intramolecular bromoetherication reaction.arrow_forwardDraw the resonance contributor for the following compound: CH3-C=O-NH2arrow_forward
- 1. Draw the structure of A and B 2. Draw the mechanism of the reactionarrow_forward(a) Explain why an alkylamine is more basic than ammonia?(b) How would you convert(i) Aniline to nitrobenzene (ii) Aniline to iodobenzenearrow_forwardNot all aldehyde give a positve Bendicts test. Which of the follwing aldehydes do? a. d. b. e. c.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
