
Concept explainers
One of the steps in the pentose phosphate pathway for glucose catabolism is the reaction of sedoheptulose 7-phosphate with glyceraldehydes 3-phosphate in the presence of a transaldolase to yield erythrose 4-phosphate and fructose 6-phosphate.
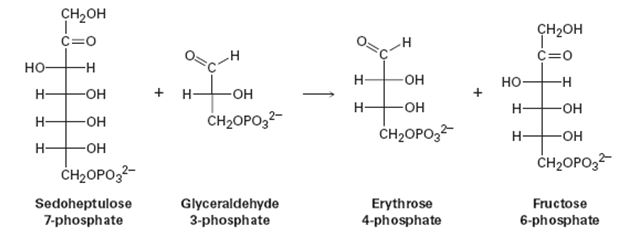
(a) The first part of the reaction is the formation of a protonated Schiff base of sedoheptulose 7-phosphate with a lysine residue in the enzyme followed by a retro-aldol cleavage to give an enamine plus erythrose 4-phosphate. Show the structure of the enamine and the mechanism by which it is formed.
(b) The second part of the reaction is a nucleophilic addition of the enamine to glyceraldehyde 3-phosphate followed by hydrolysis of the Schiff base to give fructose 6-phosphate. Show the mechanism.
Trending nowThis is a popular solution!

Chapter 29 Solutions
ORGANIC CHEMISTRY-EBOOK>I<
Additional Science Textbook Solutions
Basic Chemistry (5th Edition)
Organic Chemistry
Chemistry: Structure and Properties
General, Organic, & Biological Chemistry
Organic Chemistry
Chemistry & Chemical Reactivity
- One of the steps in the pentose phosphate pathway for glucose catabolism is the reaction of xylulose 5-phosphate with ribose 5-phosphate in the presence of a transketolase to give glyceraldehyde 3-phosphate and sedoheptulose 7-phosphate. (a) The first part of the reaction is nucleophilic addition of thiamin diphosphate (TPP) ylide to xylulose 5-phosphate, followed by a retro-aldol cleavage to give glyceraldehyde 3-phosphate and a TPPcontaining enamine. Show the structure of the enamine and the mechanism by which it is formed. (b) The second part of the reaction is addition of the enamine to ribose 5-phosphate followed by loss of TPP ylide to give sedoheptulose 7-phosphate. Show the mechanism.arrow_forward(a) Are galactose and mannose constitutional isomers or stereoisomers? (b) Draw the structure of galactose 1-phosphate and mannose 6-phosphate. (c) Are these two phosphates constitutional isomers or stereoisomers?arrow_forwardIn the glycolytic pathway, a six-carbon sugar (fructose 1,6-bisphosphate) is cleaved to form two three-carbon sugars, which undergo further metabolism . In this pathway, an isomerization of glucose 6-phosphate tofructose 6-phosphate (shown below) occurs two steps before the cleavage reaction (the intervening step is phosphorylation of fructose 6-phosphate to fructose 1,6-bisphosphate ). What does the isomerization step accomplish from a chemical perspective? (Hint: Consider what might happen if the C—C bond cleavage were to proceed without the preceding isomerization.)arrow_forward
- (i) Write the product obtained when D-glucose reacts with HCN. (ii) What type of bonding stabilizes the α-helix structure of proteins? (iii) Write the name of the disease caused by the deficiency of vitamin B12arrow_forwardCarbonic anhydrase facilitates the dissolution of carbon dioxide gas into water by catalyzing its hydration. Is the catalyzed reaction efficient? By what criterion do you state that?arrow_forwardIdentify the organic functional group and reaction type for the following reaction. The reactant is a(n) - carboxylic acid hexose - Aldohexose - aldotetrose -deoxyhexose -carboxylic acid tetrose - ketohexose The product is a(n) - carboxylic acid tetrose - aldotetrose -alcohol hexose -aldohexose -carboxylic acid hexose - alcohol tetrose The reaction type is - hemiacetal formation -hydrolysis -oxidation( Benedict’s) -acetal formation -reduction( hydrogenation) - mutarotationarrow_forward
- The dehydration of citrate to yield cis-aconitate, a step in the citric acid cycle, involves the pro-R “arm’’ of citrate rather than the pro-S arm. Which of the following two products is formed?arrow_forwardIf an enzyme-catalyzed reaction has a high rate at low pH and low rate at higher pH, this implies that a group on either the enzyme or the substrate must be for an efficient reaction. leaving group oxidoreductase coenzymes O protonated deprotonated The compound that consists of deoxyribose linked by an N-glycosidic bond to N-9 of guanine is: adenylate deoxyguanosine guanosine nucleotide guanylatearrow_forwardCholesterol-lowering drugs commonly target O HMG-COA synthase, the enzyme that catalyzes the synthesis of mevalonate O pyruvate decarboxylase complex, which catalyzes the synthesis of acetyl-CoA O mevalonate, an isoprene O HMG-COAreductase, the rate-limiting enzyme in cholesterol biosynthesis O HMG-CoA synthase, the enzyme that catalyzes the first step in cholesterol biosynthesisarrow_forward
- what is the structure digested by and how many reducing sugars are present ОН ОН Но OH HO Но HO- OH HO, The trisaccharide pictured could be completely digested (ie into monomers) by a beta-galactosidase and an alpha-glucosidase an alpha-galactosidase and sucrase lactase and sucrase a beta-fructosidase and lactasearrow_forwardStep 7 of the citric acid cycle is shown. Which statement best describes what occurs in this step? CO₂ 1 CH || CH + H₂O CO₂ fumarate CO₂™ fumarase HO C-H CH₂ CO₂ malate A) Fumarate undergoes hydrogenation with hydrogens and electrons provided by the enzyme fumarase. B) Fumarate undergoes hydration with the aid of the enzyme fumarase. C) Fumarate undergoes hydrolysis with the aid of the enzyme fumarase. D) Fumarate undergoes reduction with the aid of the cofactor fumarase.arrow_forwardConsider the structure of raffinose, a trisaccharide found in sugar beets and a number of higher plants. HO CH,OH Но- OH OCH, Но Но OH НОСН Но CH,OH ÓH raffinose (a) Classify raffinose as a reducing or nonreducing sugar, and tell how you know. (b) Identify the glycoside linkages in raffinose, and clas- sify each as either a or B. (c) Name the monosaccharides formed when raffinose is hydrolyzed in aqueous acid. (d) What products are formed when raffinose is treated with dimethyl sulfate in NaOH, and then with aqueous acid and heat?arrow_forward

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


