
3-47 Answer true or false.
(a) The name of a binary ionic compound consists of the name of the positive ion followed by the name of the negative ion.
(b) In naming binary ionic compounds, it is necessary to state the number of each ion present in the compound.
(c) The formula of aluminum oxide is AL2O3.
(d) Both copper(II) oxide and cupric oxide are acceptable names for CuO.
(e) The systematic name for Fe2O3 is iron(II) oxide.
(f) The systematic name for FeCO3 is ironcarbonate.
(g) The systematic name for NaH2PO4 is sodium dihydrogen phosphate.
(h) The systematic name for K,HPO4 is dipotassium hydrogen phosphate.
(i) The systematic name for Na20 is sodium oxide.
(j) The systematic name for PCL3 is potassium chloride.
(k) The formula of ammonium carbonate is NH4CO3.
(a)
Interpretation:
To analyse whether the given statement -The name of a binary ionic compound consists of the name of the positive ion followed by the name of the negative ion, is true or not.
Concept Introduction:
A covalent bond is formed by sharing of same number of electrons between two atoms to complete their octet. Atoms taking part in covalent bond formation may share one, two or three electron pairs thus forming single, double and triple bond respectively.
A binary covalent compound contains two kinds of atoms.
A covalent compound can be made of a atoms of the same element like Hydrogen, Oxygen etc, or it may be formed by atoms of different elements like
According to the rules for naming, binary covalent compound is named in this order-first the less electronegative element and then the more electronegative element.
Answer to Problem 3.47P
The name of a binary ionic compound consists of the name of the positive ion followed by the name of the negative ion. Thus, the statement is true.
Explanation of Solution
According to the rules for naming, binary covalent compound is named in this order-first the less electronegative element and then the more electronegative element.
For Example-
Electronic configuration of Beryllium, 2,2.
Valence shell has 2 electrons.
But to follow octet rule valance shall requires 8 electrons, for that it donates its 1 electron to two fluorine each.
On ejecting of 2 electrons now valence shell for Beryllium counts only 2 electrons, which completes its duplet. Hence it follows the octet rule.
Since, beryllium forms a cation it is written first, and Fluorine forming an anion is written later.
Therefore, the name of a binary ionic compound consists of the name of the positive ion followed by the name of the negative ion.
(b)
Interpretation:
To analyse whether the given statement -In naming binary ionic compounds, it is necessary to state the number of each ion present in the compound, is true or not.
Concept Introduction:
Binary compounds are those compounds which have atoms of two different elements. Since, the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
Binary compounds may or may not have the same ratio in all the existing atoms. For example: carbon monoxide, CO contains 1 carbon and 1 oxygen atom but carbon dioxide
Answer to Problem 3.47P
In naming binary ionic compounds, it is necessary to state the number of each ion present in the compound. Thus, the statement is true.
Explanation of Solution
Binary compounds may or may not have the same ratio in all the existing compound.
For example: carbon monoxide, CO contains 1 carbon and 1 oxygen atom but carbon dioxide
For example: In Iron (II) oxide, FeO, the ratio of Iron and Oxygen is 1:1 but in Iron (III) oxide,
Therefore. in naming binary ionic compounds, it is necessary to state the number of each ion present in the compound.
(c)
Interpretation:
To analyse whether the given statement -The formula of aluminium oxide is
Concept Introduction:
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
Answer to Problem 3.47P
The formula of aluminium oxide is
Explanation of Solution
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences. The naming of ionic compounds is done by crisscross method. Here, Al has +3 charge and oxygen has -2 charge thus, there will be 2 aluminum atoms and 3 oxygen atoms in the compound.
The formula of aluminium oxide is
(d)
Interpretation:
To analyse whether the given statement -Both copper (II) oxide and cupric oxide are acceptable names for
Concept Introduction:
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
Answer to Problem 3.47P
Both copper (II) oxide and cupric oxide are acceptable names for
Explanation of Solution
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
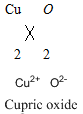
Therefore, both copper (II) oxide and cupric oxide are acceptable names for
(e)
Interpretation:
To analyse whether the given statement -The systematic name for
Concept Introduction:
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
Answer to Problem 3.47P
The systematic name for
Explanation of Solution
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
Iron (II) oxide.
The systematic name for
The systematic name for
(f)
Interpretation:
To analyse whether the given statement -The systematic name for
Concept Introduction:
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
Answer to Problem 3.47P
The systematic name for
Explanation of Solution
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
The systematic name for
(g)
Interpretation:
To analyse whether the given statement -The systematic name for
dihydrogen phosphate is true or not.
Concept Introduction:
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
Answer to Problem 3.47P
The systematic name for
Explanation of Solution
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
The systematic name for
(h)
Interpretation:
To analyse whether the given statement -The systematic name for
Concept Introduction:
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
Answer to Problem 3.47P
The systematic name for
Explanation of Solution
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
The systematic name for
(i)
Interpretation:
To analyse whether the given statement -The systematic name for
Concept Introduction:
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
Answer to Problem 3.47P
The systematic name for
Explanation of Solution
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
The systematic name for
(j)
Interpretation:
To analyse whether the given statement -The systematic name for
Concept Introduction:
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
Answer to Problem 3.47P
The systematic name for
Explanation of Solution
A binary compound may or may not have same ratio of the atoms of the elements in the existing compounds.
The ratio of Phosphorous and chlorine in Phosphorous (III) Chloride is 1:3 Therefore, systematic name for
(k)
Interpretation:
To analyse whether the given statement -The formula of ammonium carbonate is
Concept Introduction:
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
Answer to Problem 3.47P
The formula of ammonium carbonate is
Explanation of Solution
Binary compounds are those compounds which have atoms of two different elements. Since the compounds are different, there is some difference in the electronegativity in the atoms and generally formation of a polar covalent bond takes place.
In ionic compounds, there formula is calculated by multiplication of valences.
The formula of ammonium carbonate is
Want to see more full solutions like this?
Chapter 3 Solutions
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
- 3-46 Which formulas are not correct? For each that is not correct, write the correct formula. (a) Calcium oxide; CaO2 (b) Lithium oxide; LiO (c) Sodium hydrogen phosphate; NaHPO4 (d) Ammonium nitrate; NH4NO3arrow_forward2-98 Explain how the ionization energy of atoms changes when proceeding down a group of the Periodic Table and explain why this change occurs.arrow_forward3-41 Describe the structure of sodium chloride in the solid state.arrow_forward
- 2-59 You are presented with a Lewis dot structure of element X as X.. To which two groups in the Periodic Table might this element belong?arrow_forward3-45 Which formulas are not correct? For each that is not correct, write the correct formula. (a) Ammonium phosphate; (NH4 )2PO4 (b) Barium carbonate; Ba2CO3 (c) Aluminum sulfide; Al2S3 (d) Magnesium sulfide; MgSarrow_forward3-48 Potassium chloride and potassium bicarbonate are used as potassium dietary supplements. Write the formula of each compound.arrow_forward
- 3-63 What is the difference between (a) a bromine atom, (b) a bromine molecule, and (c) a bromide ion? Draw the Lewis structure for each.arrow_forward3-119 Perchloroethylene, which is a liquid at room temperature, is one of the most widely used solvents for commercial dry cleaning. It is sold for this purpose under several trade names, including Perciene®. Does this molecule have polar bonds? Is it a polar molecule? Does it have a dipole?arrow_forward3-78 Nitrous oxide, N20, laughing gas, is a colorless, nontoxic, tasteless, and odorless gas. It is used as an inhalation anesthetic in dental and other surgeries. Because nitrous oxide is soluble in vegetable oils (fats), it is used commercially as a propellant in whipped toppings Nitrous oxide dissolves in fats. The gas is added under pressure to cans of whipped topping. When the valve is opened, the gas expands, thus expanding (whipping) the topping and forcing it out of the can. (a) How many valence electrons are present in a molecule of N20? (b) Write two equivalent contributing structures for this molecule. The connectivity in nitrous oxide is NNO. (c) Explain why the following is not an acceptable contributing structure:arrow_forward
- 3-77 Ozone, O3, is an unstable blue gas with a characteristic pungent odor. In an ozone molecule, the connectivity of the atoms is OOO and both OO bonds are equivalent. (a) How many valence electrons must be present in an acceptable Lewis structure for an ozone molecule? (b) Write two equivalent resonance contributing structures for ozone. Be certain to show any positive or negative charges that may be present in your contributing structures. By equivalent contributing structures, we mean that each has the same pattern of bonding. (c) Show by the use of curved arrows how the first of your contributing structures may be converted to the second. (d) Based on your contributing structures, predict the OOO bond angle in ozone. (e) Explain why the following is not an acceptable contributing structure for an ozone molecule:arrow_forward3-43 Write the formula for the compound formed from the following pairs of ions: (a) Iron(III) ion and hydroxide ion (b) Barium ion and chloride ion (c) Calcium ion and phosphate ion (d) Sodium ion and permanganate ionarrow_forward3-26 Table 3-2 shows the following ions of copper: Cu+ and Cu2+. Do these violate the octet rule? Explain.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
