
Concept explainers
(a)
Interpretation:
The Lewis structure for the compound
(a)
Explanation of Solution
The given compound is
Step.1 Draw a skeletal structure of the molecule, arranging the atoms in their most probable order.
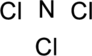
Step.2 Determine the number of valence electrons on each atom and add them to arrive at the total for the compound.
Step.3 Use the electron pairs to connect the central atom, nitrogen to each chlorine atom with a single bond.
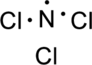
Step.4. Distribute the electrons around the terminal atoms in pairs:
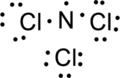
Step.5 Lewis structure of
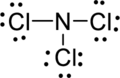
(b)
Interpretation:
The Lewis structure for the compound
(b)
Explanation of Solution
The given compound is
Step.1 Draw a skeletal structure of the molecule, arranging the atoms in their most probable order.
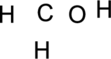
Step.2 Determine the number of valence electrons on each atom and add them to arrive at the total for the compound.
Step.3 Use the electron pairs to connect the central atom, carbon to each hydrogen and oxygen atom with a single bond.
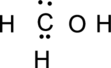
Step.4. Distribute the electrons around the terminal atoms in pairs:
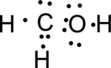
Step.5 Lewis structure of
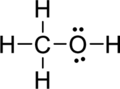
(c)
Interpretation:
The Lewis structure for the compound
(c)
Explanation of Solution
The given compound is
Step.1 Draw a skeletal structure of the molecule, arranging the atoms in their most probable order.
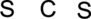
Step.2 Determine the number of valence electrons on each atom and add them to arrive at the total for the compound.
Step.3 Use the electron pairs to connect the central atom, carbon to each hydrogen and oxygen atom with a single bond.
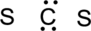
Step.4. Distribute the electrons around the terminal atoms in pairs:
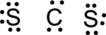
Step.5 Lewis structure of
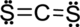
(d)
Interpretation:
The Lewis structure for the compound
(d)
Explanation of Solution
The given compound is
Step.1 Draw a skeletal structure of the molecule, arranging the atoms in their most probable order.
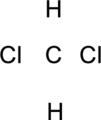
Step.2 Determine the number of valence electrons on each atom and add them to arrive at the total for the compound.
Step.3 Use the electron pairs to connect the central atom, carbon to each hydrogen and oxygen atom with a single bond.
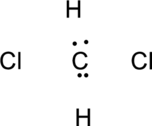
Step.4. Distribute the electrons around the terminal atoms in pairs:
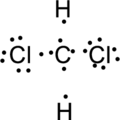
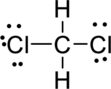
Step.5 Lewis structure of
Want to see more full solutions like this?
Chapter 3 Solutions
Package: Loose Leaf General, Organic, And Biochemistry With Connect 2-semester Access Card
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





