
Principles of General, Organic, Biological Chemistry
2nd Edition
ISBN: 9780073511191
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 3.83AP
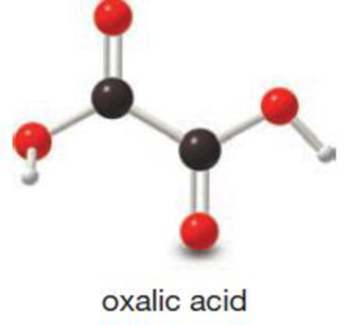
Convert the 3-D model of oxalic acid into a Lewis structure and include all nonbonded electron pairs on atoms that contain them. Oxalic acid occurs naturally in spinach and rhubarb. Although oxalic acid is toxic, you would have to eat about nine pounds of spinach at one time to ingest a fatal dose.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Convert the 3-D model of oxalic acid into a Lewis structure and include all nonbonded electron pairs on atoms that contain them. Oxalic acid occurs naturally in spinach and rhubarb. Although oxalic acid is toxic, you would have to eat about nine pounds of spinach at one time to ingest a fatal dose.
Convert the 3-D model of the general anesthetic methoxyfl urane into a Lewis structure and include all nonbonded electron pairs on atoms that contain them.
Complete Table 3 (Remember that the values pertain to the Central atoms only. In this table, you will have more than one central atom so report more than one value, ie. 3 / 3)
Table 3 Molecules with Multiple Central Atoms
Molecule
C2H4
H2O2.
CH3OH.
CH3NH2
Number of Bond Pairs
/
/
/
/
Number of Lone Pairs
/
/
/
/
Number of Electron Domains
/
/
/
/
Molecular Geometry (Shape)
/
/
/
/
Bond Angle
/
/
/
/
Polar or Non-Polar
Nonpolar
Polar
Polar
Polar
Chapter 3 Solutions
Principles of General, Organic, Biological Chemistry
Ch. 3.1 - Predict whether the bonds in the following species...Ch. 3.2 - Write the ion symbol for an atom with the given...Ch. 3.2 - Prob. 3.4PCh. 3.2 - Prob. 3.5PCh. 3.2 - How many electrons and protons are contained in...Ch. 3.2 - Prob. 3.7PCh. 3.3 - Write the formula for the ionic compound formed...Ch. 3.3 - Prob. 3.9PCh. 3.4 - Prob. 3.10PCh. 3.4 - Give the symbol for each ion. a. stannous b....
Ch. 3.4 - Name each ionic compound. a. NaF b. MgO c. SrBr2...Ch. 3.4 - Name each ionic compound. a. CrCl3 b. PbS c. SnF4...Ch. 3.4 - Prob. 3.14PCh. 3.5 - List four physical properties of ionic compounds.Ch. 3.6 - Write the formula for the compound formed when K+...Ch. 3.6 - Prob. 3.17PCh. 3.6 - Name each compound. a. Na2CO3 b. Ca(OH)2 c....Ch. 3.6 - Prob. 3.19PCh. 3.7 - Use electron-dot symbols to show how a hydrogen...Ch. 3.7 - Prob. 3.21PCh. 3.8 - Draw a Lewis structure for each covalent molecule....Ch. 3.8 - Prob. 3.23PCh. 3.8 - Prob. 3.24PCh. 3.9 - Prob. 3.25PCh. 3.9 - Prob. 3.26PCh. 3.10 - Prob. 3.27PCh. 3.11 - Prob. 3.28PCh. 3.11 - Prob. 3.29PCh. 3.11 - Show the direction of the dipole in each bond....Ch. 3.12 - Prob. 3.31PCh. 3.12 - Prob. 3.32PCh. 3 - Which formulas represent ionic compounds and which...Ch. 3 - Which pairs of elements are likely to form ionic...Ch. 3 - Prob. 3.35UKCCh. 3 - Prob. 3.36UKCCh. 3 - Prob. 3.37UKCCh. 3 - Prob. 3.38UKCCh. 3 - Prob. 3.39UKCCh. 3 - Prob. 3.40UKCCh. 3 - Prob. 3.41UKCCh. 3 - Prob. 3.42UKCCh. 3 - Prob. 3.43UKCCh. 3 - Prob. 3.44UKCCh. 3 - Prob. 3.45UKCCh. 3 - Prob. 3.46UKCCh. 3 - (a) Translate each ball-and-stick model to a Lewis...Ch. 3 - Prob. 3.48UKCCh. 3 - Prob. 3.49APCh. 3 - How many protons and electrons are present in each...Ch. 3 - Prob. 3.51APCh. 3 - Prob. 3.52APCh. 3 - Prob. 3.53APCh. 3 - Give the ion symbol for each ion. a. barium ion b....Ch. 3 - Prob. 3.65APCh. 3 - Write the formula for the ionic compound formed...Ch. 3 - Prob. 3.67APCh. 3 - Prob. 3.68APCh. 3 - Name each ionic compound. a. Na2O b. BaS c. PbS2...Ch. 3 - Name each ionic compound. a. KF b. ZnCl2 c. Cu2S...Ch. 3 - Prob. 3.71APCh. 3 - Write formulas to illustrate the difference...Ch. 3 - Prob. 3.73APCh. 3 - Name each ionic compound. a. (NH4)2SO4 b. NaH2PO4...Ch. 3 - Prob. 3.75APCh. 3 - Prob. 3.76APCh. 3 - Prob. 3.77APCh. 3 - Label each statement as true or false. Correct any...Ch. 3 - Prob. 3.79APCh. 3 - Prob. 3.80APCh. 3 - Prob. 3.81APCh. 3 - Prob. 3.82APCh. 3 - Convert the 3-D model of oxalic acid into a Lewis...Ch. 3 - Convert the 3-D model of the general anesthetic...Ch. 3 - Prob. 3.85APCh. 3 - Prob. 3.86APCh. 3 - Prob. 3.87APCh. 3 - Prob. 3.88APCh. 3 - Prob. 3.89APCh. 3 - Prob. 3.90APCh. 3 - Prob. 3.91APCh. 3 - Prob. 3.92APCh. 3 - Prob. 3.93APCh. 3 - Prob. 3.94APCh. 3 - Rank the atoms in each group in order of...Ch. 3 - Prob. 3.96APCh. 3 - Prob. 3.97APCh. 3 - Prob. 3.98APCh. 3 - Prob. 3.99APCh. 3 - Which bond in each pair is more polarthat is, has...Ch. 3 - Prob. 3.101APCh. 3 - Prob. 3.102APCh. 3 - Isobutyl cyanoacrylate is used in medical glues to...Ch. 3 - Prob. 3.104APCh. 3 - Prob. 3.105CPCh. 3 - Prob. 3.106CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the correct Lewis structure for the following molecules so4-2 OH-arrow_forward1. In its Lewis dot symbol, which element would have four (4) dots? Select one: a.Si b.F c.N d.S 2. Which compound is expected to be soluble in a polar solvent? Select one: a.CH4 b.NH3 c.None of these. d.BF3 3. Which compound is nonpolar? Select one: a.CO2 b.None of these. c.HCl d.H2O 4. In HCN, the formal charge on nitrogen is Select one: a.+1 b.0 c.–1 d.None of these. 5. For the carbonate anion, CO3–2, the number of contributing equienergetic resonance structures is Select one: a.3 b.4 c.1 d. 2arrow_forwardThe compound XeCl2F2 can exist in two different forms. One form is polar and the other form is non-polar. Draw a valid Lewis structure of XeCl2F2. Then, draw two 3-dimensional representations of this molecule (including all lone pairs); one that shows the polar form with a correct dipole arrow and the other that shows the non-polar form of the molecule.arrow_forward
- Draw an acceptable Lewis structure for each compound, assuming the atoms are connected as arranged. Formaldehyde (H2CO) is a preservative, and glycolic acid (HOCH2CO2H) is used to make dissolving sutures ?arrow_forwardDraw an acceptable Lewis structure for each compound, assuming the atoms are connected asarranged. Hydrogen cyanide (HCN) is a poison, formaldehyde (H2CO) is a preservative, and glycolic acid (HOCH2CO2H) is used to make dissolving sutures.arrow_forwardWhat is wrong with this Lewis dot structure for the H₂CO molecule? :Ö: 0- I H-C-Harrow_forward
- How do you know when to draw a solid wedge vs a dashed wedge when drawing 3D bond-line structures? I know that solid-wedge means the atom is pointing towards you and dashed wedge means it's in the back, but how do you know which atoms are in the front as opposed to the back? How can you tell what the configuration will look like in space just by looking at the lewis structure or name?arrow_forwardDraw a Lewis Structure For CH4O. Polar or nonpolar molecule? Draw also a 3D structure (entire molecule) for CH4Oarrow_forwardDraw Lewis structures for each of the following molecules: (a) CH5N (contains a bond between C and N); (b) CH3NO2 (contains a bond between C and N but no bonds between C and O); (c) CH2O; (d) CH2Cl2; (e) BrCNarrow_forward
- Draw a valid Lewis structure for each molecule. a. HI b. CH 2F 2 c. H 2Se d. C 2Cl 6arrow_forwardDraw 2 resonance structures for the following polyatomic anion: NCO– The C atom is bonded directly to the N atom and the O atom in this linear molecule. Be sure to show all lone pairs and nonzero formal charges present in each resonance structure that you draw. Each structure should only have one atom with a nonzero formal charge. Clearly indicate which of the 2 nonequivalent resonance structures that you draw is LEAST important.arrow_forwardDraw out the organic shorthand for the molecules below, and then draw another possible resonance structure of the same molecule next to with arrows to show electron movement. -CH3CH2CONH3 -CH3CH2COOHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Types of bonds; Author: Edspira;https://www.youtube.com/watch?v=Jj0V01Arebk;License: Standard YouTube License, CC-BY