
Solutions Manual for Organic Chemistry
10th Edition
ISBN: 9781259636387
Author: Carey Dr., Francis A
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 3, Problem 49DSP
Interpretation Introduction
Interpretation:
The orientation of the
Concept introduction:
The basic chair conformation of cyclohexane is shown below:
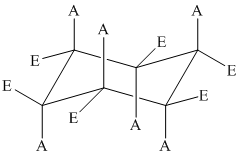
The substituents on alternate carbon atoms, which are on the same side of the ring, have the same orientation – either axial or equatorial.
Two substituents are cis to each other if they are on the same side of the ring.
Two substituents are trans to each other if they are on the opposite side of the ring.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A second category of six-carbon carbohydrates, called ketohexoses, has the constitution shown. How many stereoisomeric 2-ketohexoses are possible?
True or false
4-bromoaniline could also be named o-bromoaniline.
A typical unsaturated fatty acid has a trans double bond in its structure.
Trytophan is a hydrophillic, non-polar amino acid.
Glucose is a substrate.
The substrate for the enzyme succinate dehydrogenase is succinate.
If hydrogen bonding between amino acids in the same polypeptide give a coiled shape to the protein we have an example of primary protein structure.
A salt bridge would be the type of interaction between two leucines in a tertiary protein structure.
Isomerases catalyze the bonding of molecules using ATP energy.
True or False
Beta (1 -> 4) glycosidic linkages are prevalent in carbohydrates considered to be more digestible, such as amylose and maltose
Chapter 3 Solutions
Solutions Manual for Organic Chemistry
Ch. 3.1 - Identify the alkanes corresponding to each of the...Ch. 3.1 - Find the conformations in Figure 3.4 in which the...Ch. 3.2 - Sketch a potential energy diagram for rotation...Ch. 3.2 - Acetylcholine is a neurotransmitter in the central...Ch. 3.2 - Prob. 5PCh. 3.5 - The heats of combustion of ethylcyclopropane and...Ch. 3.8 - Prob. 7PCh. 3.10 - The following questions relate to a cyclohexane...Ch. 3.10 - Draw the most stable conformation of...Ch. 3.11 - Prob. 10P
Ch. 3.11 - Prob. 11PCh. 3.12 - Based on what you know about disubstituted...Ch. 3.12 - Write structural formulas for the most stable...Ch. 3.14 - Cubane (C4H8) is the common name of the polycyclic...Ch. 3.14 - Prob. 15PCh. 3.14 - Prob. 16PCh. 3.14 - Prob. 17PCh. 3.14 - Prob. 18PCh. 3.15 - Prob. 19PCh. 3 - Give the IUPAC names of each of the following: (a)...Ch. 3 - Draw Newman projections for the gauche and...Ch. 3 - Identify all atoms that are (a) anti and (b)...Ch. 3 - Prob. 23PCh. 3 - Prob. 24PCh. 3 - Prob. 25PCh. 3 - Prob. 26PCh. 3 - Prob. 27PCh. 3 - Prob. 28PCh. 3 - Oxidation of 4-tert-butylthiane proceeds according...Ch. 3 - The following are representations of two forms of...Ch. 3 - Draw (a) a Newman projection of the most stable...Ch. 3 - Write a structural formula for the most stable...Ch. 3 - Sight down the C-2-C-3 bond, and draw Newman...Ch. 3 - Prob. 34PCh. 3 - Sketch an approximate potential energy diagram for...Ch. 3 - Prob. 36PCh. 3 - Even though the methyl group occupies an...Ch. 3 - Which do you expect to be the more stable...Ch. 3 - Arrange the trimethylcyclohexane isomers shown in...Ch. 3 - Identify the more stable stereoisomer in each of...Ch. 3 - One stereoisomer of 1,1,3,5-tetramethylcyclohexane...Ch. 3 - One of the following two stereoisomers is...Ch. 3 - In each of the following groups of compounds,...Ch. 3 - The heats of combustion of the more and less...Ch. 3 - The measured dipole moment of ClCH2CH2Cl is 1.12D....Ch. 3 - Prob. 46PCh. 3 - Prob. 47PCh. 3 - Prob. 48DSPCh. 3 - Prob. 49DSPCh. 3 - Prob. 50DSPCh. 3 - Prob. 51DSPCh. 3 - Prob. 52DSPCh. 3 - Prob. 53DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The structure of 4 isomers of an aldotetrose carbohydrate are given. 1) select every structure that is a diastereomer of structure D A, B, or C? 2) select every structure that is a enantiomer of structure C D, B, or A? 3) select every structure that is a stereoisomer of structure D A, B, or Carrow_forwardA What of the following is true about the cyclic structure of scheme A? (a) Pyranose ring ; b) Furanose ring ; c) Alpha anomeric configuration ; d) a and c ; e) b and carrow_forwardLinolenic acid and stearidonic acid are omega-3 fatty acids, unsaturated fatty acids that contain the first double bond located at C3, when numbering begins at the methyl end of the chain. Predict how the melting point of stearidonic acid compares with the melting points of linolenic and stearic acids. A current avenue of research is examining the use of soybean oil enriched in stearidonic acid as a healthier alternative to vegetable oils that contain fewer degrees of unsaturation.arrow_forward
- 1. A carbohydrate has the molecular formula C6H12O6 and has three chiral carbons. Is this a ketohexose, an aldohexose, or an aldopentose? Explain2. Show the Fischer projection formula for the organic product of the reaction between D-ribose and warm, dilute nitric acid. Circle the carbons that are oxidized in this reaction.arrow_forwardWhen a pyranose is in the chair conformation in which the CH2OH group and the C-1 OH group are both in axial positions, the two groups can react to form an acetal. This is called the anhydro form of the sugar (it has “lost water”). The anhydro form of D-idose is shown here. Explain why about 80% of d-idose exists in the anhydro form in an aqueous solution at 100 °C, but only about 0.1% of D-glucose exists in the anhydro form under the same conditions.arrow_forwardWhich has the greater polarizability? Explain.(a) Br- or I- (b) CH2 = CH2 or CH3—CH3 (c) H2O or H2Searrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning