
Concept explainers
Magnesium ribbon reacts with acid to produce hydro- gen gas and magnesium ions. Different masses of magnesium ribbon are added to 10 mL of the acid. The volume of the hydrogen gas obtained is a measure of the number of moles of hydrogen produced by the reaction. Various measurements are given in the table below.
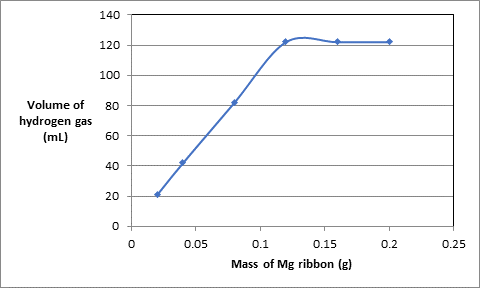
(a) Draw a graph of the results by plotting the mass of Mg versus the volume of the hydrogen gas.
(b) What is the limiting reactant in experiment 1?
(c) What is the limiting reactant in experiment 3?
(d) What is the limiting reactant in experiment 6?
(e) Which experiment uses stoichiometric amounts of each reactant?
(f) What volume of gas would be obtained if 0.300 g of Mg ribbon were used? If 0.010 g were used?
(a)
Interpretation:.
The graph between mass of Mg versus the volume of hydrogen gas should be plotted..
Concept introduction:.
The number of moles of a substance is related to mass and molar mass as follows:.
Here,mis mass andMis molar mass of the substance..
The density of solution can be calculated as follow:.
Here, m is mass and V is volume.
Answer to Problem 74QAP

Explanation of Solution
The data of mass of Mg ribbon in grams and volume of hydrogen gas produced in experiments is as follows:.
| Experiment | Mass of Mg ribbon (g) | Volume of acid used (mL) | Volume of hydrogen gas (mL) |
| 1 | 0.020 | 10.0 | 21 |
| 2 | 0.040 | 10.0 | 42 |
| 3 | 0.080 | 10.0 | 82 |
| 4 | 0.120 | 10.0 | 122 |
| 5 | 0.160 | 10.0 | 122 |
| 6 | 0.200 | 10.0 | 122 |
To plot put the data of mass of Mg ribbon on x-axis and volume of hydrogen gas at y-axis:.
(b)
Interpretation:
The limiting reactant in experiment 1 should be determined..
Concept introduction:.
The number of moles of a substance is related to mass and molar mass as follows:.
Here,mis mass andMis molar mass of the substance..
The density of solution can be calculated as follow:.
Here, m is mass and V is volume.
Answer to Problem 74QAP
Mg is limiting reactant.
Explanation of Solution
The balanced chemical reaction will be as follows:.
According to experiment 1, mass of Mg ribbon is 0.020 g, volume of acid used is 10.0 mL and volume of
The density of
Putting the values,
Molar mass of
From the balanced chemical reaction, 1 mol of hydrogen gas is produced from 1 mol of Mg thus, number of moles of Mg required to produce
The mass of Mg is 0.020 g and molar mass of Mg is 24.305 g/mol thus, number of moles of Mg will be:.
Since, number of moles of Mg required is
(c)
Interpretation:
The limiting reactant in experiment 3 should be determined..
Concept introduction:.
The number of moles of a substance is related to mass and molar mass as follows:.
Here,mis mass andMis molar mass of the substance..
The density of solution can be calculated as follow:.
Here, m is mass and V is volume.
Answer to Problem 74QAP
Mg is limiting reactant.
Explanation of Solution
The balanced chemical reaction will be as follows:.
According to experiment 3, mass of Mg ribbon is 0.080 g, volume of acid used is 10.0 mL and volume of
The density of
Putting the values,
Molar mass of
From the balanced chemical reaction, 1 mol of hydrogen gas is produced from 1 mol of Mg thus, number of moles of Mg required to produce
The mass of Mg is 0.080 g and molar mass of Mg is 24.305 g/mol thus, number of moles of Mg will be:.
Since, number of moles of Mg required is
(d)
Interpretation:
The limiting reactant in experiment 6 should be determined..
Concept introduction:.
The number of moles of a substance is related to mass and molar mass as follows:.
Here,mis mass andMis molar mass of the substance..
The density of solution can be calculated as follow:.
Here, m is mass and V is volume.
Answer to Problem 74QAP
Acid is limiting reactant.
Explanation of Solution
The balanced chemical reaction will be as follows:.
According to experiment 6, mass of Mg ribbon is 0.200 g, volume of acid used is 10.0 mL and volume of
The density of
Putting the values,
Molar mass of
From the balanced chemical reaction, 1 mol of hydrogen gas is produced from 1 mol of Mg thus, number of moles of Mg required to produce
The mass of Mg is 0.200 g and molar mass of Mg is 24.305 g/mol thus, number of moles of Mg will be:.
Since, number of moles of Mg required is
(e)
Interpretation:
The experiment that uses stoichiometric amounts of each reactant should be determined..
Concept introduction:.
The number of moles of a substance is related to mass and molar mass as follows:.
Here,mis mass andMis molar mass of the substance..
The density of solution can be calculated as follow:.
Here, m is mass and V is volume.
Answer to Problem 74QAP
Experiment 4.
Explanation of Solution
According to balance chemical reaction, 1 mol of Mg gives 1 mol of hydrogen gas thus, the experiment in which same number of moles of Mg reacts with acid to form hydrogen gas that experiment uses stoichiometric amounts of each reactant..
This cannot be experiment 1, 3 and 6 because ratio of number of moles of Mg and hydrogen gas is not 1:1 in these experiments..
Check experiment 2: mass of Mg is 0.040 g and molar mass of Mg is 24.305 g/mol thus, number of mol of Mg will be:
The volume of
The density of
Putting the values,
Molar mass of
The number of moles of Mg and hydrogen gas is not same thus, it is not experiment 2..
Check experiment 4: mass of Mg is 0.120 g and molar mass of Mg is 24.305 g/mol thus, number of mol of Mg will be:
The volume of
The density of
Putting the values,
Molar mass of
The number of moles of Mg and hydrogen gas is approximately same thus, it is experiment 4..
Check experiment 5: mass of Mg is 0.160 g and molar mass of Mg is 24.305 g/mol thus, number of mol of Mg will be:
The volume of
The density of
Putting the values,
Molar mass of
The number of moles of Mg and hydrogen gas is not same thus, it is not experiment 4..
Therefore, experiment 4 uses stoichiometric amounts of each reactant.
(f)
Interpretation:
The volume of the gas for 0.300 g and 0.010 g of Mg ribbon should be calculated.
Concept introduction:.
The number of moles of a substance is related to mass and molar mass as follows:.
Here,mis mass andMis molar mass of the substance..
The density of solution can be calculated as follow:.
Here, m is mass and V is volume.
Answer to Problem 74QAP
The volume of hydrogen gas produced from 0.120 g of Mg and 0.010 g of Mg is 122 mL and 11.32 mL respectively.
Explanation of Solution
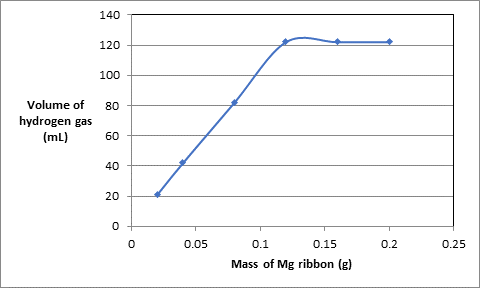
The graph between mass of Mg ribbon and volume of hydrogen gas is as follows:.

According to the graph, above the mass of Mg 0.120 g, the volume of hydrogen gas becomes constant at 122 mL thus, the volume of hydrogen gas produced if 0.120 g of Mg is burned will be 122 mL.
Considering only the straight line in the graph,.
| Experiment | Mass of Mg ribbon (g) | Volume of acid used (mL) | Volume of hydrogen gas (mL) |
| 1 | 0.020 | 10.0 | 21 |
| 2 | 0.040 | 10.0 | 42 |
| 3 | 0.080 | 10.0 | 82 |
| 4 | 0.120 | 10.0 | 122 |
The plot will be as follows:.

Comparing this with equation of straight line
For the mass of ribbon 0.010 g, the volume of hydrogen gas can be calculated as follows:.
Therefore, the volume of hydrogen gas is 11.32 mL.
Want to see more full solutions like this?
Chapter 3 Solutions
Owlv2, 4 Terms (24 Months) Printed Access Card For Masterton/hurley's Chemistry: Principles And Reactions, 8th
- When potassium chlorate is subjected to high temperatures, it decomposes into potassium chloride and oxygen. (a) Write a balanced equation for the decomposition. (b) In this decomposition, the actual yield is 83.2%. If 198.5 g of oxygen are produced, how much potassium chlorate decomposed?arrow_forward3.115 The average person exhales 1.0 kg of carbon dioxide in a day. Describe how you would estimate the number of CO2 molecules exhaled per breath for this average person.arrow_forwardWrite an equation from the following description: reactants are gaseous NH3 and O2, products are gaseous NO2 and liquid H2O, and the stoichiometric coefficients are 4, 7, 4, and 6, respectively.arrow_forward
- Fig. 5-5 illustrates a schematic diagram of a combustion device used to analyze organic compounds. Given that a certain amount of a compound containing carbon, hydrogen, and oxygen is combusted in this device, explain how the data relating to the mass of CO2 produced and the mass of H2O produced can be manipulated to determine the empirical formula.arrow_forwardIf 7.7*10^25 molecules of CO2 are produced in a combustion reaction, what is the mass in kg of CO2 that is produced?arrow_forwardA student was synthesizing Aspirin (C9H8O4) in the laboratory produced by the reaction of salicylic acid (C7H6O3) and acetic anhydride (C4H6O3).Using 4.0 g of salicylic acid as the limiting reactant, determine the theoretical yield. When the student weighed the aspirin product on the balance, the mass was 7.44g.C7H6O3(s) + C4H6O3(l) → C9H8O4(s) + CH3CO2H(aq)[Molar masses: 138.1 102.1a) What is the actual yield of aspirin?b) What is the theoretical yield of aspirin?c) Calculate the percent yield for this synthesis?arrow_forward
- You are given a crushed sample that is a mixture of limestone (calcium carbonate), lime (calcium oxide), and sand. The calcium carbonate, or limestone, is the only material present in the mixture that will decompose when heated. You subject a 6.0685 g sample of the mixture to strong heating and after the sample reaches constant mass (no more mass is lost with additional heating), the sample has a final weight of 3.9247 g. What is the percentage of calcium carbonate present in the original mixture? (MW of calcium carbonate = 100.1 g/mol) Equation for reaction = CaCO3(s) -> CaO(s) + CO2(g).arrow_forwardIron(II) sulfate forms several hydrates with the general formula FeSO4·xH2O, where x is an integer. If the hydrate is heated, the water can be driven off, leaving pure FeSO4 behind. Suppose a sample of a certain hydrate is heated until all the water is removed, and it's found that the mass of the sample decreases by45.%. Which hydrate is it? That is, what is x?arrow_forward4. Imagine that you are given 0.2500 g of a sample of copper(II) sulfate pentahydrate (CuSO4 • 5 H2O). You very carefully heat the compound for an extended period of time to drive off water, after which you determine the mass of the remaining solid to be 0.1598 g. (i) Determine whether the data given confirm the formula of the hydrate. You must (ii) show any relevant calculations that support your answer.arrow_forward
- Ammonia can be generated by heating together the solids NH4Cl and Ca(OH)2. Other products of the reaction include CaCl2 and H2O. Initially a mixture of 33.0 g each of NH4Cl and Ca(OH)2 was heated. - If the calculated percent yield was 98.27%, what is the experimental mass of the ammonia obtained?arrow_forward1.54 gg H2H2 is allowed to react with 9.77 gg N2N2, producing 1.41 gg NH3NH3. What is the theoretical yield in grams for this reaction under the given conditions? Express your answer to three significant figures and include the appropriate units.arrow_forwardMany home barbecues are fueled with propane gas (C3H8). What mass of carbon dioxide is produced upon the complete combustion of 14.5 L of propane.?Assume that the density of the liquid propane in the tank is 0.621g/mL(express answer in kilograms)arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





