
Concept explainers
MATHEMATICAL Predict the predominant forms of the amino acids from Question 8 at pH 10.
Interpretation:
The predominant forms of amino acids, histidine, asparagine, tryptophan, proline, and tyrosine, at pH 10 are to be predicted.
Concept introduction:
The name ‘amino acids’ itself implies that they have the amino group and carboxylic acid group. The central atom of the amino acid is called the alpha-carbon and that is why all the amino acids are called alpha-amino acids.
The naturally occurring amino acids are alpha-amino acids. The amino group attains the positive charge and the carboxyl group attains the negative charge at the neutral pH (potential of hydrogen).
Answer to Problem 9RE
Solution:
The imidazole ring of the histidine amino acid is deprotonated and the
The
The
The
The
Explanation of Solution
The imidazole ring in the side chain of the histidine amino acid remains partially protonated in the physiological condition, which classifies the amino acid as the positively charged amino acid and keeps this amino acid in the polar amino acid category.
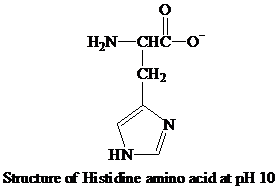
The histidine loses the proton ions from the imidazole ring and the
The

When the pH increases and reaches to 10, the
The
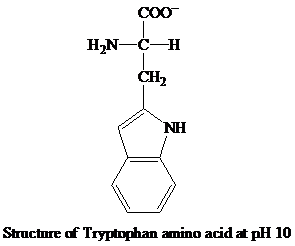
Then, the amino group becomes neutral and the carboxyl group attains the negative charge. The negative charge of the carboxyl group contributes to the anionic behavior of the tryptophan amino acid at the pH 10.
The proline remains positively-charged at the acidic pH as the amino group is having the proton ion, and as the pH increases, the deprotonation occurs and the carboxyl group loses the proton ion and becomes negatively-charged.

The positive charge and the negative charge cancel out the effect of each other and thus, the whole amino acid becomes neutral in nature at the pH 10.
The tyrosine has the phenyl group in the side chain and hydroxyl group is also present at the phenyl group. The phenolic hydroxyl is 50% mixture of the protonated and deprotonated forms.

The
Therefore, it can be concluded that the histidine becomes anionic, asparagine becomes anionic, tryptophan becomes anionic, proline becomes neutral, and tyrosine becomes anionic at the pH 10.
(a)
Anion
(b)
Anion
(c)
Anion
(d)
Neutral
(e)
Anion
Explanation:
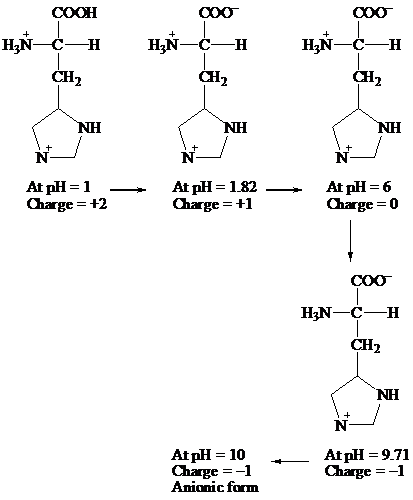
COOH COO- COO-
׀ ׀ ׀
H3N+ — C — H H3N+ — C — H H3N+ — C — H
׀ ׀ ׀
CH2 CH2 CH2
׀ ׀ ׀

At pH = 1 → At pH= 1.82 → At pH = 6
Charge = +2 Charge = +1 Charge = 0
↓
COO-
H3N— C — H
׀
CH2
׀

At pH = 10 ← At pH = 9.71
Charge = -1 Charge = -1
Anionic form
Hence, the predominant form of histidine at pH 10 is anionic.
___________________________________________________________________________
(b)
Given information: Asparagine at pH 10
Explanation:
COOH COO- COO-
׀ ׀ ׀
H3N+ — C — H H3N+ — C — H H2N — C — H
׀ | |
CH2 CH2 CH2
׀ ׀ ׀
C = O C = O C = O
׀ ׀ ׀
NH2 NH2 NH2
At pH = 1 → At pH= 2.02 → At pH = 8.80 → At pH = 10
Charge = +1 Charge = 0 Charge = -1 Charge = -1
Anionic
form
Hence, the predominant form of asparagine at pH 10 is anionic.
___________________________________________________________________________
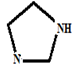
(c)
Given information: Tryptophan at pH 10
Explanation:
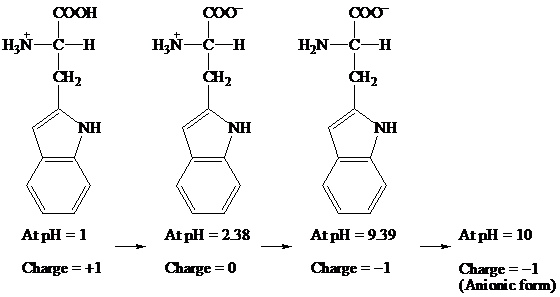
COOH COO- COO-
׀ ׀ ׀
H3N+ — C — H H3N+ — C — H H2N — C — H
׀ ׀ ׀
CH2 CH2 CH2
׀ ׀ ׀


At pH = 1 → At pH= 2.38 → At pH = 9.39 → At pH = 10
Charge = +1 Charge = 0 Charge = -1 Charge = -1
(Anionic form)
Hence, the predominant form of tryptophan at pH 10 is anionic.
___________________________________________________________________________
(d)
Given information: Proline at pH 10.
Explanation:
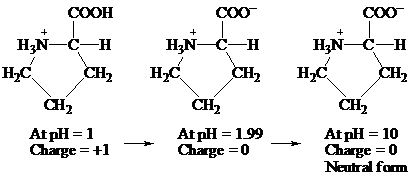
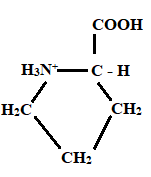
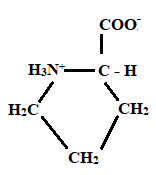

At pH = 1 → At pH= 1.99 → At pH = 10
Charge = +1 Charge = 0 Charge = 0
Neutral form
Hence, the predominant form of proline at pH 10 is neutral.
___________________________________________________________________________
(e)
Given information: Tyrosine at pH 10.
Explanation:
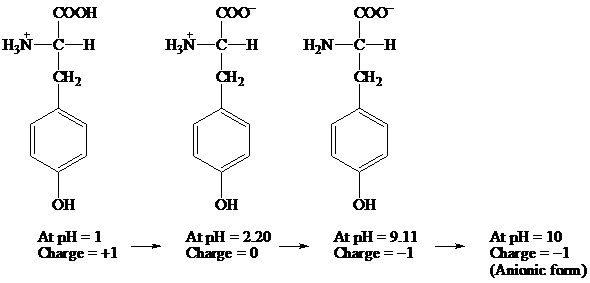
COOH COO- COO-
׀ ׀ ׀
H3N+ — C — H H3N+ — C — H H2N — C — H
׀ ׀ ׀
CH2 CH2 CH2
׀ ׀ ׀


At pH = 1 → At pH= 2.20 → At pH = 9.11 → At pH = 10
Charge = +1 Charge = 0 Charge = -1 Charge = -1
(Anionic form)
Hence, the predominant form of tyrosine at pH 10 is anionic.
Want to see more full solutions like this?
Chapter 3 Solutions
Biochemistry
- REFLECT AND APPLY Suggest a reason why the cell membranes of bacteria grown at 20C tend to have a higher proportion of unsaturated fatty acids than the membranes of bacteria of the same species grown at 37C. In other words, the bacteria grown at 37C have a higher proportion of saturated fatty acids in their cell membranes.arrow_forwardREFLECT AND APPLY Comment on the energetics of protein folding in light of the information in this chapter.arrow_forwardREFLECT AND APPLY Reflect on the evolutionary implications of the structural similarities and functional differences of cytochromes on the one hand and hemoglobin and myoglobin on the other.arrow_forward
- REFLECT AND APPLY Comment on the evolutionary implications of the differences in the genetic code observed in mitochondria.arrow_forwardREFLECT AND APPLY What is the importance of pyrophosphatase in the synthesis of nucleic acids?arrow_forwardREFECT AND APPLY What might you infer (or know) about the stability of amino acids, when compared with that of other building- block units of biopolymers (sugars, nucleotides, fatty acids, etc.)?arrow_forward
- REFLECT AND APPLY Suggest an explanation for the observation that when proteins are chemically modified so that specific side chains have a different chemical nature, these proteins cannot be denatured reversibly.arrow_forwardREFLECT AND APPLY Is amino acid activation energetically favored? Why or why not?arrow_forwardREFLECT AND APPLY Immature rats are fed all the essential amino acids but one. Six hours later they are fed the missing amino acid. The rats fail to grow. Explain this observation.arrow_forward
- REFLECT AND APPLY The process of protein folding is spontaneous in the thermodynamic sense. It gives rise to a highly ordered conformation that has a lower entropy than the unfolded protein. How can this be?arrow_forwardREFLECT AND APPLY Experimental evidence strongly suggests that the protein portions of cytochromes have evolved more slowly (as judged by the number of changes in amino acids per million years) than the protein portions of hemoglobin and myoglobin and even more slowly than hydrolytic enzymes. Suggest a reason why.arrow_forwardREFLECT AND APPLY The amino acid hydroxyproline is found in collagen. There is no codon for hydroxyproline. Explain the occurrence of this amino acid in a common protein.arrow_forward
 BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
