
Organic Chemistry - Standalone book
10th Edition
ISBN: 9780073511214
Author: Francis A Carey Dr., Robert M. Giuliano
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3.14, Problem 14P
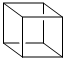
Cubane (C4H8) is the common name of the polycyclic hydrocarbon shown. As its nameimplies, its structure is that of a cube. How many rings are present in cubane according tothe bond disconnection rule?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
) How many stereogenic double bonds are in octa-1,3,5-triene? How many stereocentersare there?
Calculate the number of degrees of unsaturation in a compound ofmolecular formula C4H6, and propose possible structures ?
How many rings and π bonds are contained in a compound of molecularformula C8H12 that is hydrogenated to a compound of molecular formulaC8H14?
Chapter 3 Solutions
Organic Chemistry - Standalone book
Ch. 3.1 - Identify the alkanes corresponding to each of the...Ch. 3.1 - Find the conformations in Figure 3.4 in which the...Ch. 3.2 - Sketch a potential energy diagram for rotation...Ch. 3.2 - Acetylcholine is a neurotransmitter in the central...Ch. 3.2 - Prob. 5PCh. 3.5 - The heats of combustion of ethylcyclopropane and...Ch. 3.8 - Prob. 7PCh. 3.10 - The following questions relate to a cyclohexane...Ch. 3.10 - Draw the most stable conformation of...Ch. 3.11 - Prob. 10P
Ch. 3.11 - Prob. 11PCh. 3.12 - Based on what you know about disubstituted...Ch. 3.12 - Write structural formulas for the most stable...Ch. 3.14 - Cubane (C4H8) is the common name of the polycyclic...Ch. 3.14 - Prob. 15PCh. 3.14 - Prob. 16PCh. 3.14 - Prob. 17PCh. 3.14 - Prob. 18PCh. 3.15 - Prob. 19PCh. 3 - Give the IUPAC names of each of the following: (a)...Ch. 3 - Draw Newman projections for the gauche and...Ch. 3 - Identify all atoms that are (a) anti and (b)...Ch. 3 - Prob. 23PCh. 3 - Prob. 24PCh. 3 - Prob. 25PCh. 3 - Prob. 26PCh. 3 - Prob. 27PCh. 3 - Prob. 28PCh. 3 - Oxidation of 4-tert-butylthiane proceeds according...Ch. 3 - The following are representations of two forms of...Ch. 3 - Draw (a) a Newman projection of the most stable...Ch. 3 - Write a structural formula for the most stable...Ch. 3 - Sight down the C-2-C-3 bond, and draw Newman...Ch. 3 - Prob. 34PCh. 3 - Sketch an approximate potential energy diagram for...Ch. 3 - Prob. 36PCh. 3 - Even though the methyl group occupies an...Ch. 3 - Which do you expect to be the more stable...Ch. 3 - Arrange the trimethylcyclohexane isomers shown in...Ch. 3 - Identify the more stable stereoisomer in each of...Ch. 3 - One stereoisomer of 1,1,3,5-tetramethylcyclohexane...Ch. 3 - One of the following two stereoisomers is...Ch. 3 - In each of the following groups of compounds,...Ch. 3 - The heats of combustion of the more and less...Ch. 3 - The measured dipole moment of ClCH2CH2Cl is 1.12D....Ch. 3 - Prob. 46PCh. 3 - Prob. 47PCh. 3 - Prob. 48DSPCh. 3 - Prob. 49DSPCh. 3 - Prob. 50DSPCh. 3 - Prob. 51DSPCh. 3 - Prob. 52DSPCh. 3 - Prob. 53DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- skeletal line bond drawing of C6H5CO2H with an aromatic 6 membered ringarrow_forwardDraw the structure(s) of all of the alkene isomers, C5H10, that contain a branched chain. Consider E/Z stereochemistry of alkenes.arrow_forwardCycloheptatrienyl radical (C7H7∙) contains a cyclic, completely conjugated system of π electrons. Is it aromatic? Is it antiaromatic? Explain.arrow_forward
- Draw all 12 acyclic (no rings) isomers of formula C4H7Br. Include stereoisomers.arrow_forwardIs the methyl group at the junction of rings C and D axial or equatorial to ring C?arrow_forwardDraw all the isomers with molecular formula C6H12 that contain a cyclobutane ring. (Hint: There are seven.)arrow_forward
- (a) How many stereogenic double bonds are in octa-1,3,5-triene? How many stereocenters are there? Draw and name the four stereoisomers of octa-1,3,5-triene.arrow_forwardHow many rings and π(pi) bonds are contained in compound A and draw one possible structure for this compound A. Compound A has molecular formula C6H10 and is hydrogenated to a compound having molecular formula C6H12arrow_forward(A)Menthol, used to flavor various foods and tobacco, is the most stable stereoisomer of 2-isopropyl-5-methylcyclohexanol. Draw its most stable conformation. Is the hydroxyl group cis or trans to the isopropyl group? To the methyl group? (b) Neomenthol is a stereoisomer of menthol. That is, it has the same constitution but differs in the arrangement of its atoms in space. Neomenthol is the second most stable stereoisomer of 2-isopropyl-5methylcyclohexanol; it is less stable than menthol but more stable than any other stereoisomer. Write the structure of neomenthol in its most stable conformation.arrow_forward
- The compounds drawn should each contain a cyclohexane ring. For all three compounds draw a wedge and dash structure, Chair I, and Chair II conformations. Formula: C9H18 with substitution 1,1- disubstituted with stereochemistry of (R,S) Formula: C7H13Cl with substitution 1,3- disubstituted with stereochemistry of (R,R) Formula: C7H14O with substitution 1,4- disubstituted with stereochemistry (S,S)arrow_forwardthe number of elements of unsaturation in the molecular formula C4H6. Giveall nine possible structures having this formula. Remember thata double bond = one element of unsaturationa ring = one element of unsaturationa triple bond = two elements of unsaturationarrow_forwardFive isomeric alkanes (A–E) having the molecular formula C6H14 are each treated with Cl2 + hv to give alkyl halides having molecular formulaC6H13Cl. A yields five constitutional isomers. B yields four constitutionalisomers. C yields two constitutional isomers. D yields threeconstitutional isomers, two of which possess stereogenic centers. Eyields three constitutional isomers, only one of which possesses astereogenic center. Identify the structures of A–E.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License