
ORGANIC CHEMISTRY-EBOOK>I<
9th Edition
ISBN: 9781305084414
Author: McMurry
Publisher: INTER CENG
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 3.3, Problem 10P
Interpretation Introduction
a) Two tertiary carbons
Interpretation:
The structures of the
Concept introduction:
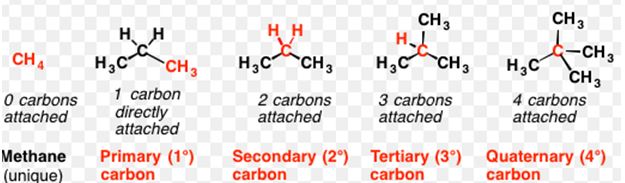
- Primary carbons are carbons attached to one carbon.
- Secondary carbons are attached to two other carbons.
- Tertiary carbons are attached to three other carbons.
- Finally, quaternary carbons are attached to four other carbons.
Interpretation Introduction
b) Isopropyl group
Interpretation:
The structures of the alkanes containing the following groups are to be drawn.
Concept introduction:
- Primary carbons are carbons attached to one carbon.
- Secondary carbons are attached to two other carbons.
- Tertiary carbons are attached to three other carbons.
- Finally, quaternary carbons are attached to four other carbons.

Interpretation Introduction
c) One quaternary and one secondary carbons
Interpretation:
The structures of the alkanes containing the following groups are to be drawn.
Concept introduction:
- Primary carbons are carbons attached to one carbon.
- Secondary carbons are attached to two other carbons.
- Tertiary carbons are attached to three other carbons.
- Finally, quaternary carbons are attached to four other carbons.

Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Write structural formulas for compounds that meet the following descriptions:(a) An alkene, C6H12, that cannot have cis–trans isomersand whose longest chain is 5 carbons long(b) An alkene with a chemical formula of C10H12 that hascis–trans isomers and contains a benzene ring.
(a) What structural feature is associated with each type of hydrocarbon: alkane, cycloalkane, alkene, and alkyne?(b) Give the general formula for each type.(c) Which hydrocarbons are considered saturated?
Indicate whether each statement is true or false. (a) Twogeometric isomers of pentane are n-pentane and neopentane.(b) Alkenes can have cis and trans isomers around theCC double bond. (c) Alkynes can have cis and trans isomersaround the CC triple bond.
Chapter 3 Solutions
ORGANIC CHEMISTRY-EBOOK>I<
Ch. 3.1 - Prob. 1PCh. 3.1 - Prob. 2PCh. 3.1 - Identify the functional groups in the following...Ch. 3.2 - Draw structures of the five isomers of C6H14.Ch. 3.2 - Propose structures that meet the following...Ch. 3.2 - Prob. 6PCh. 3.3 - Draw the eight 5-carbon alkyl groups (pentyl...Ch. 3.3 - Identify the carbon atoms in the following...Ch. 3.3 - Prob. 9PCh. 3.3 - Prob. 10P
Ch. 3.4 - Give IUPAC names for the following compounds:Ch. 3.4 - Prob. 12PCh. 3.4 - Name the eight 5-carbon alkyl groups you drew in...Ch. 3.4 - Give the IUPAC name for the following hydrocarbon,...Ch. 3.7 - Make a graph of potential energy versus angle of...Ch. 3.7 - Sight along the C2-C1 bond of 2-methylpropane...Ch. 3.7 - Sight along the C2-C3 bond of 2,3-dimethylbutane,...Ch. 3.7 - Draw a Newman projection along the C2-C3 bond of...Ch. 3.SE - Prob. 19VCCh. 3.SE - Prob. 20VCCh. 3.SE - Draw a Newman projection along the C2-C3 bond of...Ch. 3.SE - Prob. 22APCh. 3.SE - Prob. 23APCh. 3.SE - Propose structures for the following: (a) A...Ch. 3.SE - Prob. 25APCh. 3.SE - Draw the structures of the following molecules:...Ch. 3.SE - Draw structures that meet the following...Ch. 3.SE - Prob. 28APCh. 3.SE - In each of the following sets, which structures...Ch. 3.SE - There are seven constitutional isomers with the...Ch. 3.SE - Prob. 31APCh. 3.SE - Draw compounds that contain the following: (a) A...Ch. 3.SE - Prob. 33APCh. 3.SE - Draw and name all monochloro derivatives of...Ch. 3.SE - Draw structures for the following: (a)...Ch. 3.SE - Prob. 36APCh. 3.SE - Draw a compound that: (a) Has nine primary...Ch. 3.SE - Give IUPAC names for the following compounds:Ch. 3.SE - Name the five isomers of C6H14.Ch. 3.SE - Explain why each of the following names is...Ch. 3.SE - Prob. 41APCh. 3.SE - Consider 2-methylbutane (isopentane). Sighting...Ch. 3.SE - What are the relative energies of the three...Ch. 3.SE - Construct a qualitative potential-energy diagram...Ch. 3.SE - Prob. 45APCh. 3.SE - Draw the most stable conformation of pentane,...Ch. 3.SE - Draw the most stable conformation of...Ch. 3.SE - Prob. 48APCh. 3.SE - Prob. 49APCh. 3.SE - Formaldehyde, H2C=O, is known to all biologists...Ch. 3.SE - Prob. 51APCh. 3.SE - Increased substitution around a bond leads to...Ch. 3.SE - Prob. 53APCh. 3.SE - In the next chapter we'll look at...Ch. 3.SE - We’ll see in the next chapter that there are two...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Cyclopropane (C3H6, a three-membered ring) is more reactive than most other cycloalkanes.(a) Draw a Lewis structure for cyclopropane.(b) Compare the bond angles of the carbon atoms in cyclopropane with those in an acyclic (noncyclic) alkane.(c) Suggest why cyclopropane is so reactive.arrow_forwardThis question is about the chemistry of alkenes, which are unsaturated hydrocarbons. (a) State what is meant by the term unsaturated as applied to a hydrocarbon. (1) (b) An organic compound, X, is an unsaturated hydrocarbon with molecular formula CH₂. (i) Draw the displayed formulae and give the names of two molecules with molecular formula C₂H, which are E/Z isomers. (3) Isomer 1 Isomer 2 Name: Name:arrow_forwardA certain hydrocarbon has a molecular formula of C5H8. Which of the following is not a structural possibility for this hydrocarbon: (d) It contains an alkyne O It contains one ring and one double bond (c) It contains two double bonds and no rings O (b) It contains one ring and no double bondsarrow_forward
- A saturated alkanol X has the relative molecular mass of 74.0. (a) Give the general formula of alkanol. (b)(i) Deduce the molecular formula of X. (Relative atomic mass of C=12, H=1, O=16) (c)(i) X is optically active. Draw the two enantiomers of X. (ii) Name X.arrow_forwardDraw structures for the following molecules. (a) 2-iodo-2,3-dimethylheptane (b) 4-cyclopropyl-3-ethyl-2-methyloctane (c) 1-ethyl-2,4-dimethylcyclopentanearrow_forwardAnswer true or false. (a) Alkenes and alkynes are nonpolar molecules. (b) The physical properties of alkenes are similar to those of alkanes of the same carbon skeletons. (c) Alkenes that are liquid at room temperature are insoluble in water and when added to water, will float on water.arrow_forward
- A certain hydrocarbon has a molecular formula of C5H8. Which of the following is not a structural possibility for this hydrocarbon? (a) It is a cycloalkane. (b) It contains one ring and one double bond. (c) It contains two double bonds and no rings. (d) It is an alkyne.arrow_forward1. (A) DRAW are all the possible isomers for dibromobutane and the cylic isomers for C3H4Br2 (B) WRITE THE STRUCTURAL OR CONDENSED FORMULAR FOR C3H7Br C3H6Br2 C3H5Br3 C5H11Brarrow_forwardDraw structures corresponding to the following IUPAC names: (a) 2-Methyl-1,5-hexadiene (b) 3-Ethyl-2,2-dimethyl-3-heptene (c) 2,3,3-Trimethyl-1,4,6-octatriene (d) 3,4-Diisopropyl-2,5-dimethyl-3-hexenearrow_forward
- (a) The compound given below had the following IUPAC name and structural formula dibromocyclopentane C3H6CHBrCHBr (i) What type of isomerism is possible in the organic compound? (ii) Draw all the pairs of possible isomers and name them.arrow_forwardGive the molecular, structural and displayed formulae of the following molecules: (a) 2,2-dimethylpropane (b) 3-bromo-2, 4, 4-trimethyloctanearrow_forwardTRUE OR FALSE (a) Both ethylene and acetylene are planar molecules. (b) An alkene in which each carbon of the double bond has two different groups bonded to it will show cis-trans isomerism. (c) Cis-trans isomers have the same molecular formula but a different connectivity of their atoms. (d) Cis-2-butene and trans -2-butene can be interconverted by rotation about the carbon–carbon double bond. (e) Cis-trans isomerism is possible only among appropriately substituted alkenes. (f) Both 2-hexene and 3-hexene can exist as pairs of cis-trans isomers. (g) Cyclohexene can exist as a pair of cis-trans isomers. (h) 1-Chloropropene can exist as a pair of cis-trans isomers.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning