
Concept explainers
Interpretation:
The number of hydrogen atoms attached to the Carbon atoms indicated in the structure of morphine as shown below has to be determined.
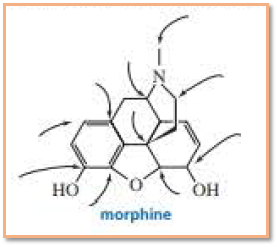
Concept Introduction:
Any organic molecule can be using certain rules by IUPAC (International Union for pure and applied chemistry). IUPAC names consist of three parts in major namely Prefix suffix and root word.
Prefix represents the substituent present in the molecule and its position In the root name.
Suffix denotes the presence of functional group if any in the molecule. It can be an
For
For Alkene suffix ‘ene’.
For Alkyne suffix ‘yne’.
Root word represents the longest continuous carbon skeleton of the organic molecule.
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Organic Chemistry - MasteringChemistry
- Complete each of the following by supplying the missing product indicated by the question mark:arrow_forwardwhat happens when pyridine is treated with nitrous acid?arrow_forwardGive IUPAC names for all saturated unbranched-chain compounds that are named as the following. a. Heptanals b. Heptanonesarrow_forward
- Which carbon atom is the hemiacetal carbon atom in each of the following structures?arrow_forwardWhich of the three compounds pentanal, 2-pentanone, and 2-pentcmol will react with each of the following oxidizing or reducing agents? There may be more than one correct answer in a given situation. a. K2Cr2O7 b. Tollens solution c. Benedicts solution d. H2, Ni catalystarrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning




