
Interpretation:
The
Concept introduction:
The anti and gauche bonds in cyclohexane are represented by sawhorse projections of staggered conformations of
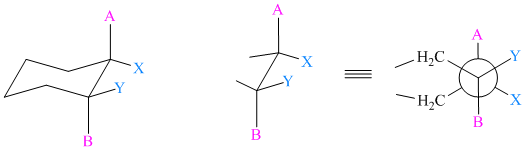
The substituents in anti-relationship are
The substituents in gauche relationship are
In the following structure, each path shows anti relationship where a substituent is equatorial.
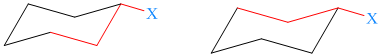
In the following structure, each path shows gauche relationship where a substituent is axial.

The torsion (dihedral) angle for eclipsed conformation is

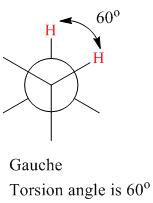
The torsion (dihedral) angle for gauche conformation is
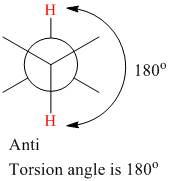
The torsion (dihedral) angle for anti-conformation is
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
EBK ORGANIC CHEMISTRY
- Provide the IUPAC name of the compound shown below (don’t forget the stereocenters).arrow_forwardWhat is the highest energy conformation of 3-methylpentane when viewed down the 2-3 carbon-carbon bond?arrow_forwardAssign relative priorities to each set of substituents: a. -CH2CH2CH3 -CH(CH3)2 -CH = CH2 -CH3 b. - CH2NH2 -NH2 - OH -CH2OH c. -C(=O)CH3 - CH =CH2 -Cl -C=Narrow_forward
- 8. Consider 1-bromo-2-methylpropane. Draw a Newman projection of the following (when viewed directlyalong the C1-C2 bond) a. staggered conformation of lowest energyb. eclipsed conformation of highest energyarrow_forwardIdentify each substituent in the following compound as axial or equatorial, and tell whether the conformation shown is the more stable or less stable chair form (green = Cl):arrow_forwardProvide the IUPAC name of the compound shown below please don’t forget to include the stereocenters.arrow_forward
- We saw that the energy cost of an axial methyl is 1.8 kcal/mol; therefore, we might expect cis-1,3-dimethylcyclohexane to have chair conformations with a difference in totalenergy of 3.6 kcal/mol. 1. Is the calculated energy difference in cis-1,3-dimethylcyclohexane higher or lower than expected? Propose an explanation for the difference between the expected energy difference and the calculated energy difference in cis-1,3-dimethylcyclohexane. (Limit your answer to 10 words or fewer) 2. Recall that an axial methyl is typically worth around 1.8 kcal/mol. Propose an explanation for the difference in the value above for 1-methyltetrahydropyran. (Limit your answer to 10 words or less) 3. Would you expect the difference in strain energy between the chairs of 5-methyl-1,3-dioxane to be greater than or less than that of 1-methyltetrahydropyran? Is it GREATER THAN or LESS THAN?arrow_forwardFor the following molecule: A. Draw both chair conformations and label substitutents as axial or equatorial B. Indicate which conformation is more stable using equilibrium arrows C. Predict the major product of the reaction with sodium methoxide D. Predict the major product of the reaction with potassium tert-butoxidearrow_forwardDraw both cis- and trans-1,4-dimethylcyclohexane in their more stable chairconformations. (a) How many stereoisomers are there of cis-1,4-dimethylcyclohexane, and how many of trans-1,4-dimethylcyclohexane? (b) Are any of the structures chiral? (c) What are the stereochemical relationships among the various stereoisomers of 1,4-dimethylcyclohexane?arrow_forward
- TRUE OR FALSE i. A =C-Br bond is more polar than a -C-Br bond. ii. A bromonium ion can undergo hydride or methyl shift to produce a more stable carbocation. iii. The two conformers of cis-1,3-diaminocyclohexane will have equal stability.arrow_forwardConvert each ball-and-stick model to a skeletal structure that clearlyshows the stereochemistry at the ring fusion of these decalin derivatives.arrow_forwardAnswer the following questions regarding a reaction shown below. For IUPAC naming, indicate absolute configurations using R/S sequence rule. For Fisher projections, all the carbons must be vertically lined up from C1 (top) to C4 (bottom). Give an IUPAC name for the starting material. Q1: Draw one of the two products of chlorination at C3 in Fisher projection. Q2: Provide the IUPAC name of Q1. Q3: Draw the other product of chlorination at C3 in Fisher projection. Q4: Provide the IUPAC name of Q3.arrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

