
EBK FOUNDATIONS OF COLLEGE CHEMISTRY
15th Edition
ISBN: 9781118930144
Author: Willard
Publisher: JOHN WILEY+SONS INC.
expand_more
expand_more
format_list_bulleted
Question
Chapter 4, Problem 1RQ
Interpretation Introduction
Interpretation:
The physical state of acetic acid at
Expert Solution & Answer
Explanation of Solution
The table is,
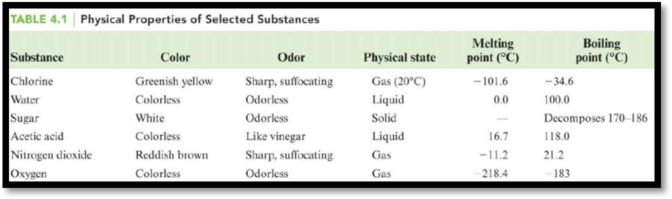
Figure 1
The boiling point of acetic acid is
The boiling point is the temperature at which a liquid boils at a fixed pressure under standard atmospheric conditions and it remains gas.
The given temperature is
The temperature in Kelvin has to be converted to Celsius.
The temperature of the acetic acid is higher than the boiling point of the acetic acid so the physical sate of the acetic acid at
Want to see more full solutions like this?
Subscribe now to access step-by-step solutions to millions of textbook problems written by subject matter experts!
Students have asked these similar questions
Consider 1n NO and NO2 and each kept in a 500mL container at 37℃.Which has a greater kinetic energy.Which moves faster?
What is the temperature in °C of 4.92 L of water at 30°C after 999.04 calories of heat have been added? (Express your answer in 2 decimal place, no unit required)
In the laboratory a student finds that it takes 48.7 calories to increase the temperature of 11.6 grams of gaseous neon from 20.3 to 38.7 degrees Celsius.
Based on these data, what is the specific heat of neon?
Chapter 4 Solutions
EBK FOUNDATIONS OF COLLEGE CHEMISTRY
Ch. 4.1 - Prob. 4.1PCh. 4.2 - Prob. 4.2PCh. 4.5 - Prob. 4.3PCh. 4.5 - Prob. 4.4PCh. 4.5 - Prob. 4.5PCh. 4 - Prob. 1RQCh. 4 - Prob. 2RQCh. 4 - Prob. 3RQCh. 4 - Prob. 4RQCh. 4 - Prob. 5RQ
Ch. 4 - Prob. 6RQCh. 4 - Prob. 7RQCh. 4 - Prob. 8RQCh. 4 - Prob. 9RQCh. 4 - Prob. 10RQCh. 4 - Prob. 11RQCh. 4 - Prob. 12RQCh. 4 - Prob. 13RQCh. 4 - Prob. 14RQCh. 4 - Prob. 15RQCh. 4 - Prob. 1PECh. 4 - Prob. 2PECh. 4 - Prob. 3PECh. 4 - Prob. 4PECh. 4 - Prob. 5PECh. 4 - Prob. 6PECh. 4 - Prob. 7PECh. 4 - Prob. 8PECh. 4 - Prob. 9PECh. 4 - Prob. 10PECh. 4 - Prob. 11PECh. 4 - Prob. 12PECh. 4 - Prob. 13PECh. 4 - Prob. 14PECh. 4 - Prob. 15PECh. 4 - Prob. 16PECh. 4 - Prob. 17PECh. 4 - Prob. 18PECh. 4 - Prob. 19PECh. 4 - Prob. 20PECh. 4 - Prob. 21PECh. 4 - Prob. 22PECh. 4 - Prob. 23AECh. 4 - Prob. 24AECh. 4 - Prob. 25AECh. 4 - Prob. 26AECh. 4 - Prob. 27AECh. 4 - Prob. 28AECh. 4 - Prob. 29AECh. 4 - Prob. 30AECh. 4 - Prob. 31AECh. 4 - Prob. 32AECh. 4 - Prob. 33AECh. 4 - Prob. 34AECh. 4 - Prob. 35AECh. 4 - Prob. 36AECh. 4 - Prob. 37AECh. 4 - Prob. 38AECh. 4 - Prob. 39AECh. 4 - Prob. 44CECh. 4 - Prob. 45CECh. 4 - Prob. 46CE
Knowledge Booster
Similar questions
- Some farmers use ammonia, NHS, as a fertilizer. This ammonia is stored in liquid form. Use the particulate perspective to show the transition from liquid ammonia to gaseous ammonia.arrow_forwardMethane, CH4, is a major component of marsh gas. When 0.5000 mol methane burns to produce carbon dioxide and liquid water, 445.1 kJ of heat is released. What is this heat in kilocalories?arrow_forwardIn a 1m3 sample of air, 3g of water that was heated so that all 3g evaporated. How many calories of heat would be required? Is heat released or consumed?arrow_forward
- When 655 J is added to a sample of ethanol, its temperature rises from 18.2 °C to 32.8 °C. What is the mass, in grams, of the ethanol sample (see Table 3.7)?arrow_forward1 calorie is equal to 4.184 joules. true or falsearrow_forwardThe ethylene glycol (d = 1.11 g/mL; c = 2.42) in a car radiator cools from 37.0°C to 25.0°C by releasing 177 kJ of heat. What volume of ethylene glycol is in the radiator?arrow_forward
- How many grams of water at 100 c could be changed from liquid to gas if 4320 calories of heat were usedarrow_forwardA certain physical process requires 36.00 kilojoules (kJ) of heat. Convert this quantity to calories.arrow_forwardHow many calories 5000 Joules are?. No computation, no pointsarrow_forward
- The freezing point of mercury is -38.8°C. What quantity of energy, in joules, is released to the surroundings if 1.90 mL of mercury is cooled from 23.0°C to -38.8°C and then frozen to a solid? (The density of liquid mercury is 13.6 g/cm3. Its specific heat capacity is 0.140 J/g·K and its heat of fusion is 11.4 J/g.)arrow_forwardA sample of mercury at 14.0°C is warmed to 128.0°C when 795 cal of heat is added. What is the mass of the mercury? (cmercury = 0.035 kcal/kg°C)arrow_forwardA 0.25g sample of a pretzel is burned. The heat it gives off is used to heat 50. g of water from 18 degrees C to 42 degrees C. What is the energy value of the pretzel, in kcal/g?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co