
Concept explainers
(a)
Interpretation:
The diagram that represents the arrangement of protons, neutrons, and electrons in
Concept introduction:
An atom is made up of three subatomic particles-neutrons, protons, and electrons. Neutron and protons are present in the nucleus of the atom, whereas electrons are revolving outside the nucleus in an atom. Isotopes are those atoms that have the same number of protons but the number of neutrons is different.
Answer to Problem 25E
The diagram that represents the arrangement of protons, neutrons, and electrons in
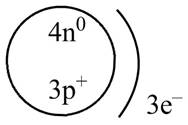
Explanation of Solution
The
The
The number of electrons and number of protons in a neutral atom is equal to its atomic number. Hence, the number of protons and number of electrons present in
The atomic mass of the element is represented by the formula as shown below.
Where,
•
•
Rearrange the above equation for the value of
Substitute the value of
One atom of
Therefore, the diagram that represents the arrangement of protons, neutrons, and electrons in
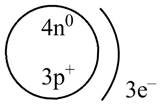
Figure 1
The diagram that represents the arrangement of protons, neutrons, and electrons in
(b)
Interpretation:
The diagram that represents the arrangement of protons, neutrons, and electrons in
Concept introduction:
An atom is made up of three subatomic particles-neutrons, protons, and electrons. Neutron and protons are present in the nucleus of the atom, whereas electrons are revolving outside the nucleus in an atom. Isotopes are those atoms that have the same number of protons but a different number of neutrons.
Answer to Problem 25E
The diagram that represents the arrangement of protons, neutrons, and electrons in
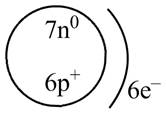
Explanation of Solution
The atomic mass of
The atomic number of
The number of electrons and number of protons in a neutral atom is equal to its atomic number. Hence, the number of protons and number of electrons present in
The atomic mass of the element is represented by the formula as shown below.
Where,
•
•
Rearrange the above equation for the value of
Substitute the value of
One atom of
Therefore, the diagram that represents the arrangement of protons, neutrons, and electrons in
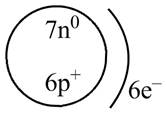
Figure 2
The diagram that represents the arrangement of protons, neutrons, and electrons in
(c)
Interpretation:
The diagram that represents the arrangement of protons, neutrons, and electrons in
Concept introduction:
An atom is made up of three subatomic particles-neutrons, protons, and electrons. Neutron and protons are present in the nucleus of the atom, whereas electrons are revolving outside the nucleus in an atom. Isotopes are those atoms that have the same number of protons but a different number of neutrons.
Answer to Problem 25E
The diagram that represents the arrangement of protons, neutrons, and electrons in
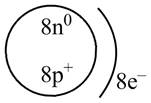
Explanation of Solution
The atomic mass of
The atomic number of
The number of electrons and number of protons in a neutral atom is equal to its atomic number. Hence, the number of protons and number of electrons present in
The atomic mass of the element is represented by the formula as shown below.
Where,
•
•
Rearrange the above equation for the value of
Substitute the value of
One atom of
Therefore, the diagram that represents the arrangement of protons, neutrons, and electrons in
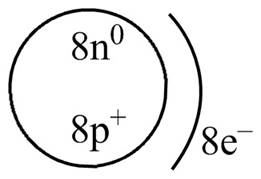
Figure 3
The diagram that represents the arrangement of protons, neutrons, and electrons in
(d)
Interpretation:
The diagram that represents the arrangement of protons, neutrons, and electrons in
Concept introduction:
An atom is made up of three subatomic particles-neutrons, protons, and electrons. Neutron and protons are present in the nucleus of the atom, whereas electrons are revolving outside the nucleus in an atom. Isotopes are those atoms that have the same number of protons but a different number of neutrons.
Answer to Problem 25E
The diagram that represents the arrangement of protons, neutrons, and electrons in
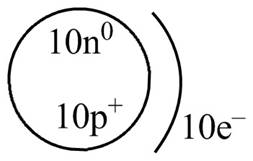
Explanation of Solution
The atomic mass of
The atomic number of
The number of electrons and number of protons in a neutral atom is equal to its atomic number. Hence, the number of protons and number of electrons present in
The atomic mass of the element is represented by the formula as shown below.
Where,
•
•
Rearrange the above equation for the value of
Substitute the value of
One atom of
Therefore, the diagram that represents the arrangement of protons, neutrons, and electrons in
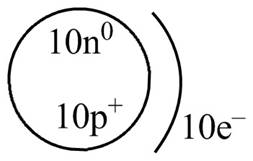
Figure 4
The diagram that represents the arrangement of protons, neutrons, and electrons in
Want to see more full solutions like this?
Chapter 4 Solutions
Modified Mastering Chemistry with Pearson eText -- Standalone Access Card -- for Introductory Chemistry: Concepts and Critical Thinking (8th Edition)
- 2-31 Tin-118 is one of the isotopes of tin. Name the isotopes of tin that contain two, three, and six more neutrons than tin-118.arrow_forward2-101 Complete the following table: Symbol Atomic number Atomic weight Mass number # of protons # of neutrons # of electrons H 0 Li 4 3 Al 26 58 78 17 20arrow_forwardGive the atomic number (Z) and the mass number (A) for each of the following: a. a carbon atom with 8 neutrons b. an aluminum atom with 14 neutrons c. an argon atom with 20 neutrons d. a copper atom with 36 neutronsarrow_forward
- 2-85 The mass of a proton is 1.67 × 10-24g. The mass of a grain of salt is 1.0 × 10-2g. How many protons would it take to have the same mass as a grain of salt?arrow_forwardUsing the information given in the table in Problem 3-35 indicate whether each of the following pairs of atoms are isotopes. a. Atom B and atom C b. Atom B and atom D c. Atom C and atom Darrow_forwardWrite the complete chemical symbol (EZA) for the isotope of copper with the following individual characteristics. a. 32 more neutrons than protons b. Two more subatomic particles than Z3066n c. The same number of neutrons as N2858i d. The same number of nucleons as Z3060narrow_forward
- 2-102 An element consists of 90.51% of an isotope with a mass of 19.992 amu, 0.27% of an isotope with a mass of 20.994 amu, and 9.22% of an isotope with a mass of 21.990 amu. Calculate the average atomic mass and identify the element.arrow_forward2-104 The average atomic weight of lithium is 6.941 amu. The two naturally occurring isotopes of lithium have the following masses: 6Li, 6.01512 amu; 7Li, 7.01600 amu. Calculate the percent abundance of 6Li and 7Li in naturally occurring lithium.arrow_forward2-69 (Chemical Connections 2A) Why does the body need sulfur, calcium, and iron?arrow_forward
- The atomic number of the element carbon (C) is 6. Write the complete chemical symbols for each of the following carbon isotopes: carbon-12, carbon-13, and carbon-14.arrow_forward2-29 How many protons and how many neutrons does each of these isotopes of radon contain? (a) Rn-210 (b) Rn-218 (c) Rn-222arrow_forward2-103 The element silver has two naturally occurring isotopes: 109Ag and 107Ag with a mass of 106.905 amu. Silver consists of 51.82% 07Ag and has an average atomic mass of 107.868 amu. Calculate the mass of 109Agarrow_forward

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





