
Concept explainers
(a)
The plot of the logarithm of the rate of recrystallization versus the inverse of the absolute temperature and to check whether it is linear or not.
Answer to Problem 4.19P
The graph of logarithm of the rate of recrystallization versus the inverse of absolute temperature is shown in figure (1) and it is linear.
Explanation of Solution
Given:
The given table is,
| S. No. | Temperature |
Time (Seconds) |
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
392 | |
| |
|
|
Calculation:
Draw the table to plot the graph of logarithm of the rate of recrystallization versus the inverse of absolute temperature.
| S. No. | Temperature |
|
Time
|
Rate
|
|
| |
|
|
|
|
|
| |
|
|
|
|
|
| |
|
|
|
|
|
| |
|
|
|
|
|
| |
392 | |
|
|
|
| |
|
|
|
|
|
The graph of logarithm of the rate of recrystallization versus the inverse of absolute temperature is shown below.
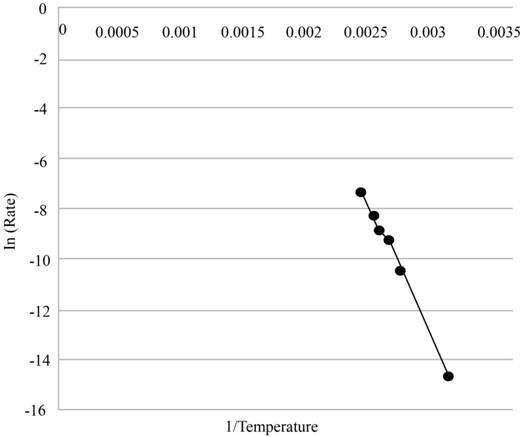
Figure (1)
Conclusion:
Therefore, the graph of logarithm of the rate of recrystallization versus the inverse of absolute temperature is shown in figure (1) and it is linear.
(b)
The activation enthalpy for the recrystallization of copper.
Answer to Problem 4.19P
The activation enthalpy for the recrystallization of copper is
Explanation of Solution
Formula Used,
The activation enthalpy for the recrystallization of copper is given by,
Here,
Calculation:
The activation enthalpy for the recrystallization of copper is calculated as,
Substitute
Conclusion:
Therefore, the activation enthalpy for the recrystallization of copper is
(c)
The reason for activation enthalpy of recrystallization of copper being less than the activation enthalpy for vacancy diffusion.
Answer to Problem 4.19P
The activation enthalpy for the recrystallization is less than the activation enthalpy for vacancy diffusion because during the process of recrystallization, the size of grains increases and the vacancy site decreases.
Explanation of Solution
Introduction:
The Gibbs free energy is the energy that is available or free, to do work under conditions of constant pressure and temperature.
The expression for the Gibb’s free energy is given by,
Here,
The term
Here,
During the process of recrystallization, the size of the grain changes to form a new crystal structure. By this process the grain size changes its size by diffusing into other grains, thereby increasing the size of the grains. When the size of the grain increases, the possibility for the vacancy site decreases; that’s why the activation enthalpy of recrystallization of copper is less than the activation enthalpy for vacancy diffusion.
Conclusion:
Therefore, the activation enthalpy for the recrystallization is less than the activation enthalpy for vacancy diffusion because during the process of recrystallization, the size of grains increases and the vacancy site decreases.
(d)
The temperature at which the copper completely crystallizes in
Answer to Problem 4.19P
The temperature at which the copper completely crystallizes in
Explanation of Solution
Formula Used,
The logarithmic rate of crystallization is given by,
Here,
Calculation:
The logarithmic rate of crystallization is calculated as,
Substitute
Drop a perpendicular from
Conclusion:
Therefore, the temperature at which the copper completely crystallizes in
Want to see more full solutions like this?
Chapter 4 Solutions
Materials Science And Engineering Properties
 Materials Science And Engineering PropertiesCivil EngineeringISBN:9781111988609Author:Charles GilmorePublisher:Cengage Learning
Materials Science And Engineering PropertiesCivil EngineeringISBN:9781111988609Author:Charles GilmorePublisher:Cengage Learning
