
Concept explainers
Interpretation:
Frequency of the first five lines in Lyman series has to be calculated.
Explanation of Solution
Frequency of First line:
Energy of photon emitted on relaxation from
Frequency of photon is calculated as shown below.
Therefore, the frequency of photon is calculated as
Wavelength of photon is calculated as shown below;
Therefore, wavelength of photon is
Frequency of Second line:
Energy of photon emitted on relaxation from
Frequency of photon is calculated as shown below.
Therefore, the frequency of photon is calculated as
Wavelength of photon is calculated as shown below;
Therefore, wavelength of photon is
Frequency of Third line:
Energy of photon emitted on relaxation from
Frequency of photon is calculated as shown below.
Therefore, the frequency of photon is calculated as
Wavelength of photon is calculated as shown below;
Therefore, wavelength of photon is
Frequency of Fourth line:
Energy of photon emitted on relaxation from
Frequency of photon is calculated as shown below.
Therefore, the frequency of photon is calculated as
Wavelength of photon is calculated as shown below;
Therefore, wavelength of photon is
Frequency of Fifth line:
Energy of photon emitted on relaxation from
Frequency of photon is calculated as shown below.
Therefore, the frequency of photon is calculated as
Wavelength of photon is calculated as shown below;
Therefore, wavelength of photon is
Summary of the wavelength of first five lines in Lyman series is given below.
| 2 | 3 | 4 | 5 | 6 | |
From the above table, in order to plot graph, the wavenumber and
Graph is plotted considering wavenumber in
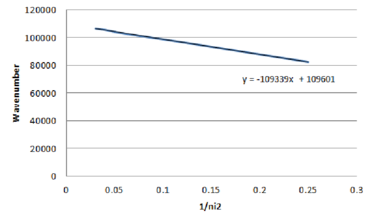
From the graph that is plotted as shown, the slope of the graph is
Want to see more full solutions like this?
Chapter 4 Solutions
GENERAL CHEMISTRY ACHIEVE ACCESS W/BOOK
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





