
(a)
Interpretation:
The three dimensional representation for
Concept introduction:
Electrons in an atom are present in particular orbitals. Sublevels contain orbitals which possess equivalent energy. Maximum two electrons are present in each orbital. The electrons present in
Answer to Problem 81E
The three dimensional representation for
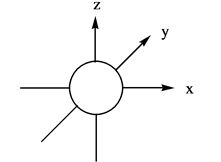
Explanation of Solution
The energy level possesses a sublevel that contains orbital. The sublevels are named as
The three dimensional representation for

Figure 1
The three dimensional representation for
(b)
Interpretation:
The three dimensional representation for
Concept introduction:
Electrons in an atom are present in particular orbitals. Sublevels contain orbitals which possess equivalent energy. Maximum two electrons are present in each orbital. The electrons present in
Answer to Problem 81E
The three dimensional representation for
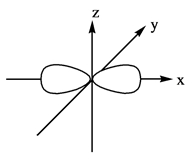
Explanation of Solution
The energy level possesses a sublevel that contains orbital. The sublevels are named as
The three dimensional representation for

Figure 2
The three dimensional representation for
(c)
Interpretation:
The three dimensional representation for
Concept introduction:
Electrons in an atom are present in particular orbitals. Sublevels contain orbitals which possess equivalent energy. Maximum two electrons are present in each orbital. The electrons present in
Answer to Problem 81E
The three dimensional representation for
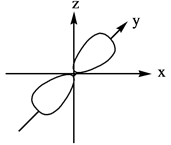
Explanation of Solution
The energy level possesses a sublevel that contains orbital. The sublevels are named as
The three dimensional representation for

Figure 3
The three dimensional representation for
(d)
Interpretation:
The three dimensional representation for
Concept introduction:
Electrons in an atom are present in particular orbitals. Sublevels contain orbitals which possess equivalent energy. Maximum two electrons are present in each orbital. The electrons present in
Answer to Problem 81E
The three dimensional representation for
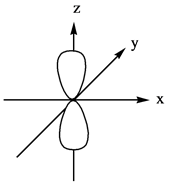
Explanation of Solution
The energy level possesses a sublevel that contains orbital. The sublevels are named as
The three dimensional representation for

Figure 4
The three dimensional representation for
Want to see more full solutions like this?
Chapter 4 Solutions
INTRODUCTORY CHEMISTRY-STD.GDE.+SOL.MAN
- Which of the following orbitals has the highest energy in a multielectron atom? The quantum numbers are given as (n, , ml). Select one: a. (5, 4, -3) b. (4, 3, −1) c. (3, 2, 0) d. (3, 0, 0) e. (4, 1, 1)arrow_forwardIn what main group(s) of the periodic table do elements have the following number of half-filled p-orbitals in the outermost principal energy level? a. 0 b. 1 c. 2 d. 3 (I'm struggling a bit so I'd like to also have an explanation of why)arrow_forwardSelect True or False: The following set of quantum numbers is correct. n = 3, l = 3, ml = 0, ms = +1/2arrow_forward
- the principal quantum number of the first d orbital is a) 1 b)2 c)3 d)4 e)5 Please help very urgent no explanation requiredarrow_forwardHow many of the elements on the current periodic table can contain orbitals having n = 1 as their principal quantum number? Enter a numerical value.arrow_forwardH2. 2. Calculate the energy of transition from n = 3 to n = 1. In what region of electromagnetic spectrum is this radiation found? Please give typed answerarrow_forward
- A photon of energy 2.5×10–18 J has a wavelength of aboutarrow_forwardHow does the wavelength of a fast-pitched baseball compare to the wavelength of an electron traveling at 110 the speed of light? What is the significance of this comparison? See Example 2-3.arrow_forwardWhat is the wavelength of the electromagnetic radiation emitted from a hydrogen atom when the electron undergoes the transition n = 4 to n = 1? In what region of the spectrum does this line occur? (See Figure 7.5.)arrow_forward
- What is the maximum number of orbitals that can be identified by each of the following sets of quantum numbers? When none is the correct answer, explain your reasoning. (a) n = 3, = 0, m = +1 (b) n = 5, = 1, (c) n = 7, = 5, (d) n = 4, = 2, m = 2arrow_forward1. The wavelength associated with an electron moving at 40% of the speed of light is 6.07 × 10−3 nm (see Example 6.5). How will the wavelength be changed if the velocity increased to 80% of the speed of light? Wavelength will be longer Wavelength will be shorter. Wavelength will not change.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





