
Interpretation: The reason for the formation of methyl m -nitrobenzoate instead of ortho and para isomers in the following reaction needs to be explained:
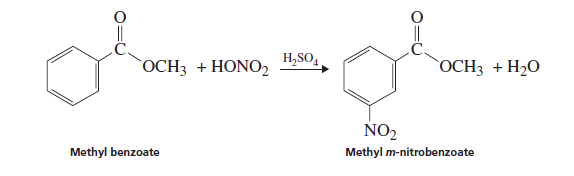
Concept Introduction: The organic reactions where an atom which is attached to an
Answer to Problem 1Q
Due to the presence of an electron-withdrawing group that is ester group (
Explanation of Solution
The methyl benzoate is less reactive towards aromatic electrophilic substitution due to the presence of an electron-withdrawing group that is the ester group,
Due to the -R effect (The withdrawal of electrons from one molecule by the other group in a molecule through delocalization is said to be a negative resonance effect (-R effect)), the
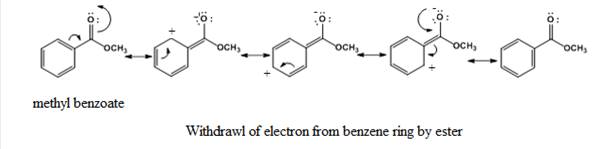
The partial positive charge is present at ortho and para position on the benzene ring and thus, it will deactivate the benzene ring towards electrophilic substitution reactions. The incoming electrophile will attack on a position where the electron density is high that is at the meta position.
Want to see more full solutions like this?
Chapter 43 Solutions
A Small Scale Approach to Organic Laboratory Techniques
- Why is it easier to remove excess acetic acid from the products than excess isopentyl alcohol?arrow_forwardFC Acylation of anisole Please write a balanced reaction scheme of the unwanted side reaction that would occur if the round bottom flask was not oven dried and contained moisture and explain how this would impact the yield of the major product.arrow_forwardWould it be favorable to get a 1,4-adduct of anthracene and maleic anhydride? Why or why not? If the 1,4-adduct of anthracene and maleic anhydride had formed, would it have different exo and endo isomers?arrow_forward
- why is acetic acid added after homogenization in extraction and isolation?arrow_forwardIf one started with ortho-ethylbenzoic acid, instead of 1,4-dimethoxybenzene what would the first reaction product (mono alkylated) formed with t-butyl alcohol be? Justify your answer (focus on relative activation of substituents).arrow_forwardWhat two β-keto esters are formed in the Dieckmann reaction of the attached diester?arrow_forward
- why it is important to keep water out of the acid-catalysed esterification of octanoic acid with 1-propanol reaction? What would happen if water got into the reaction mixture?arrow_forwardwhich statement is true in the e2 reaction of the given compounds with NaOCH2CH3?arrow_forwardWhy is a protecting group used in this reaction? Ethylene glycol can be recycled which would decrease the E-Factor of this reaction. explain why this is based on the reaction scheme above.arrow_forward
- In a few sentences, briefly explain the difference between acid catalyzed and base promoted enolation of aldehydes and ketones.arrow_forwardThe preparation of Wittig reagents requires a strong base like butyllithium. Sodium methoxide, used in this reaction to prepare the Horner-Emmons-Wittig reagent, is a weaker base than butyllithium. Suggest a reason why this weaker base is sufficient to prepare the Horner-Emmons-Wittig reagent.arrow_forwardBriefly explain why OtBu- sometimes favored over hydroxide as an elimination reagent?arrow_forward
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

