
Concept explainers
Two 10-ft3 adiabatic tanks are connected by a valve. Initially, one tank contains water at 450 psia with 10 percent quality, while the second contains water at 15 psia with 75 percent quality. The valve is now opened, allowing the water vapor from the high-pressure tank to move to the low-pressure tank until the pressure in the two becomes equal. Determine the final pressure and the final mass in each tank.
FIGURE P4–142E
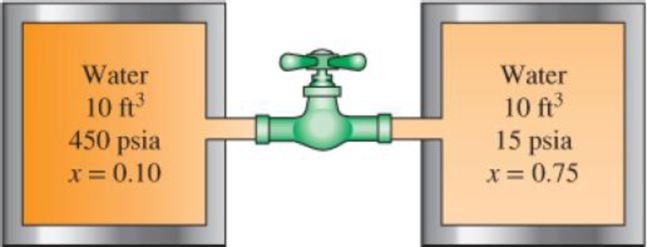
The final pressure of each tank.
The final mass of each tank.
Answer to Problem 142RP
The final pressure of each tank is
The final mass of each tank is
Explanation of Solution
Write the expression for the energy balance equation.
Here, the total energy entering the system is
Simplify Equation (V) and write energy balance relation of two adiabatic tanks.
Here, the heat to be transfer into the system is
Substitute 0 for
Here, the initial mass of tank A is
Write the expression for initial mass of tank A.
Here, the volume of the tank A is
Write the expression for initial mass of tank B.
Here, the volume of the tank B is
Write the expression for total mass of tank.
Write the expression for initial total internal energy contained in both tanks.
Write the expression for initial is equal to final specific internal energy of tank.
Determine the total volume of both the tanks.
Write the expression for initial is equal to final specific volume of tank.
Write the expression for final mass contained in both tanks.
Conclusion:
At initial pressure and quality of initial state for tank A as 450 psia and 0.10, find the value of initial specific volume and specific internal energy of the tank.
Here, the specific volume of saturated liquid for tank A is
Here, the specific internal energy of saturated liquid for tank A is
Substitute
Substitute
At initial pressure and quality of initial state for tank B as 15 psia and 0.75, find the value of initial specific volume and specific internal energy of the tank.
Here, the specific volume of saturated liquid for tank B is
Here, the specific internal energy of saturated liquid for tank B is
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
By above calculation from Table A-5E “saturated water” the final pressure of both tanks as
Thus, the final pressure of each tank is
Substitute
Thus, the final mass of each tank is
Want to see more full solutions like this?
Chapter 4 Solutions
Thermodynamics: An Engineering Approach
- Steam flows steadily through a turbine at a rate of 45,000 lbm/h, entering at 1000 psia and 900°F and leaving at 5 psia as saturated vapor. If the power generated by the turbine is 4 MW, determine the rate of heat loss from the steam.arrow_forwardWhat is the maximum volume that 3 kg of oxygen at 950 kPa and 373°C can be adiabatically expanded to in a piston–cylinder device if the final pressure is to be 100 kPa?arrow_forwardAn insulated piston–cylinder device contains 0.05 m3 of saturated refrigerant- 134a vapor at 0.8-MPa pressure. The refrigerant is now allowed to expand in a reversible manner until the pressure drops to 0.4 MPa. Determine the work done by the refrigerant.arrow_forward
- Refrigerant-134a at 320 kPa and 40°C undergoes an isothermal process in a closed system until its quality is 45 percent. On a per-unit-mass basis, determine how much work and heat transfer are required.arrow_forwardA piston–cylinder device initially contains 0.6 kg of steam with a volume of 0.1 m3 . The mass of the piston is such that it maintains a constant pressure of 800 kPa. The cylinder is connected through a valve to a supply line that carries steam at 5 MPa and 500°C. Now the valve is opened and steam is allowed to flow slowly into the cylinder until the volume of the cylinder doubles and the temperature in the cylinder reaches 250°C, at which point the valve is closed. Determine the mass of steam that has entered.arrow_forwardA 5-ft3 rigid tank initially contains refrigerant-134a at 60 psia and 100 percent quality. The tank is connected by a valve to a supply line that carries refrigerant-134a at 140 psia and 80°F. The valve is now opened, allowing the refrigerant to enter the tank, and is closed when it is observed that the tank contains only saturated liquid at 100 psia. Determine the mass of the refrigerant that entered the tank.arrow_forward
- An insulated piston–cylinder device contains 0.05 m3 of saturated refrigerant- 134a vapor at 0.8-MPa pressure. The refrigerant is now allowed to expand in a reversible manner until the pressure drops to 0.4 MPa. Determine the final temperature in the cylinder.arrow_forwardA piston-cylinder device contains steam that undergoes a reversible thermodynamic cycle. Initially, the steam is at 400 kPa and 350°C with a volume of 0.3 m3. The steam is first expanded isothermally to 150 kPa, then compressed adiabatically to the initial pressure, and finally compressed at the constant pressure to the initial state. Indicate the processes (1-2, 2-3, and 3-1) on a P-v diagram. Determine the net work and heat transfer for the cycle after you calculate the work and heat interaction for each process.arrow_forwardA 5-ft3 rigid tank initially contains refrigerant-134a at 60 psia and 100 percent quality. The tank is connected by a valve to a supply line that carries refrigerant-134a at 140 psia and 80F. The valve is now opened, allowing the refrigerant to enter the tank, and is closed when it is observed that the tank contains only saturated liquid at 100 psia. Determine (a) the mass of the refrigerant that entered the tank, (b) the amount of heat transfer with the surroundings at 708F, and (c) the entropy generated during this process.arrow_forward
- Steam is condensed in a closed system at a constant pressure of 75 kPa from a saturated vapor to a saturated liquid by rejecting heat to a thermal energy reservoir at 37°C. Determine the second-law efficiency of this process. Take T0 = 25°C and P0 = 100 kPa.arrow_forwardSteam enters an adiabatic turbine at 8 MPa and 500°C at a rate of 2.7 kg/s and leaves at 20 kPa. If the power output of the turbine is 2.5 MW, determine the temperature of the steam at the turbine exit. Neglect kinetic energy changesarrow_forwardRefrigerant-134a enters an adiabatic compressor as saturated vapor at 100 kPa at a rate of 0.7 m3 /min and exits at 1-MPa pressure. If the isentropic efficiency of the compressor is 87 percent, determine the temperature of the refrigerant at the exit of the compressor.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





