
Interpretation:
Products formed for the given reaction has to be predicted. Given reaction is shown below,
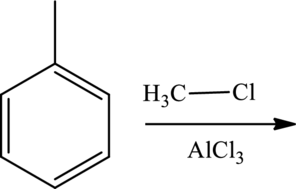
Concept Introduction:
Friedel-Crafts Alkylation: This Lewis acid-catalyzed electrophilic
Deactivators are electron withdrawing groups attached to the benzenes that have either positive charge or an atom with high electronegativity. They are meta directors.
Activators are electron donating groups attached to the benzenes that have either electron density that is able to push into benzene ring or a lone pair of electrons. They are ortho-para directing.
Halogens are deactivators that are ortho-para directing.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
EBK ORGANIC CHEMISTRY AS A SECOND LANGU
- Please help with: Predict the major product(s) expected for each of the following reactions:arrow_forwardPredict the products for each of the following reactions and propose a mechanism that explains the formation of each product.arrow_forwardProvide the correct conditions and their major products for the two reactions below:arrow_forward
