
One of the important ideas of 
- Using this device, what measurements would you need to make to test your hypothesis?
- What equations would you use in analyzing your experiment?
- Do you think you could obtain a reasonable result from a single experiment? Why or why not?
- In what way could the precision of your instruments affect the conclusions that you make?
- List ways that you could modify the equipment to improve the data you obtain if you were performing this experiment today instead of 180 years ago.
- Give an example of how you could demonstrate the relationship between heat and a form of energy other than mechanical work.
Interpretation: The balanced equations for the given reaction statements are to be identified.
Concept introduction: The relation of the work and the heat produced is calculated by the Joule experiment. The mechanical equivalent of heat is the ratio of the heat produced from the mechanical work.
(a)
To determine: The measurements required to test the hypothesis using device of Joule’s experiment.
Answer to Problem 1DE
Solution: The measurements required to test the hypothesis using device of Joule’s experiment is stated below.
Explanation of Solution
The setup required for the experiment is,
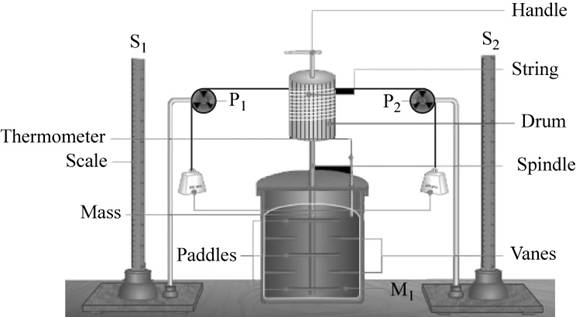
Figure 1
The relation between the work done and the heat produced is calculated in the following manner.
Using the above setup, the mechanical work is obtained by using potential energy lost by the falling mass.
Therefore, work done is equal to the potential energy of the falling mass.
Thus, the work done is calculated by the formula,
The heat that is generated in the mass of water in the calorimeter chamber is calculated by the formula,
where, water equivalent of calorimeter is the amount of water that absorbs the same amount of heat as the calorimeter to raise the temperature by one degree.
For the comparison of the work done with heat, the measurements that we need is,
- Mass of the falling object.
- Mass of water in the calorimeter.
- Height of the falling mass.
- Change in temperature.
- Water equivalent of calorimeter.
For the comparison of the work done with heat, the measurements that we need is,
- Mass of the falling object.
- Mass of water in the calorimeter.
- Height of the falling mass.
- Change in temperature.
- Water equivalent of calorimeter.
(b)
To determine: The equations used to analyze the experiment.
Answer to Problem 1DE
Solution: The equations used to analyze the experiment are stated below.
Explanation of Solution
The relation between the work done and the heat produced is to be determined.
Using the above setup, the mechanical work is obtained by using potential energy lost by the falling mass.
Therefore, work done is equal to the potential energy of the falling mass.
Thus, the work done is calculated by the formula,
The heat that is generated in the mass of water in the calorimeter chamber is calculated by the formula,
The ratio of the work done in generating heat is calculated by the formula,
Here, the constant is called as mechanical equivalent of heat.
The equations used to analyze the experiment are of mechanical work, heat and their ratio.
(c)
To determine: If the reasonable result is obtained from a single experiment.
Answer to Problem 1DE
Solution: No.
Explanation of Solution
The given experiment determines the relation of amount of work that is converted into heat energy.
This should be applicable for all sets of systems of thermodynamics.
Therefore, the same relation should be obtained in different setups of experiments.
Hence, the reasonable results are not obtained by single experiment.
The reasonable results are not obtained by single experiment.
(d)
To determine: The effect of precision of the instruments on the conclusion.
Answer to Problem 1DE
Solution: The precision in the measurement of the heat produced from work should be accurate for the perfect conclusion.
Explanation of Solution
The given experiment determines the relation of amount of work that is converted into heat energy.
The error that may occur is the loss of heat from the system. Therefore, the system should be properly isolated to measure appropriate heat.
Therefore, the precision in the measurement of the heat produced from work should be accurate for the perfect conclusion.
The precision in the measurement of the heat produced from work should be accurate for the perfect conclusion.
(e)
To determine: The modifications in the experiment that are done considering available modern amenities.
Answer to Problem 1DE
Solution: The modifications in the experiment that are done considering available modern amenities are using automated mechanical paddle stirrer and digital calorimeter.
Explanation of Solution
The mechanical work in the ancient experiment is performed by the falling masses.
Nowadays, automated mechanical paddle stirrer is available, that can be used to create mechanical work.
Also, the digital calorimeter is available that detects the change in temperature appropriately and provides an isolated system.
The modifications in the experiment that are done considering available modern amenities are using automated mechanical paddle stirrer and digital calorimeter.
(f)
To determine: The example that demonstrates the relationship between heat and a form of energy other than mechanical work.
Answer to Problem 1DE
Solution: The relationship between heat and a form of energy other than mechanical work is,
Explanation of Solution
In the above experiment, the water is stirred using a paddle with a known falling mass. The water is placed isolated in a calorimeter and a thermometer measures the temperature change in it.
The rotation in the water is obstructed by the vanes in the container. This causes the rise in temperature of water that is measured using thermometer.
The rise in temperature with the mechanical work is measured.
The relation between the work done and the heat produced is calculated in the following manner.
Using the above setup, the mechanical work is obtained by using potential energy lost by the falling mass.
Therefore, work done is equal to the potential energy of the falling mass.
Thus, the work done is calculated by the formula,
The heat that is generated in the mass of water in the calorimeter chamber is calculated by the formula,
Where, Water equivalent of calorimeter is the amount of water that absorbs the same amount of heat as the calorimeter to raise the temperature by one degree.
The ratio of the work done in generating heat is calculated by the formula,
Here, the constant is called as mechanical equivalent of heat.
Therefore, mechanical equivalent of heat is the form of energy.
The above equation is modified as,
The relationship between heat and a form of energy other than mechanical work is,
Want to see more full solutions like this?
Chapter 5 Solutions
EBK CHEMISTRY:CENTRAL SCIENCE
Additional Science Textbook Solutions
Living by Chemistry
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
General Chemistry: Atoms First
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Chemistry: Structure and Properties
Chemistry & Chemical Reactivity
- The second law of thermodynamics is sometimes paraphrased as: you can't break even. Explain. Because energy cannot be created out of nothing. Because some energy is lost in all energy transactions. Because some energy is gained in all energy transactons.arrow_forwardIn the process of isolating iron from its ores, carbon monoxide reacts with iron(III) oxide, as described by the following equation: Fe2O3(s)+3CO(g)2Fe(s)+3CO2(g)H=24.8kJ The enthalpy change for the combustion of carbon monoxide is 2CO(g)+O2(g)2CO2(g)H=566kJ Use this information to calculate the enthalpy change for the equation 4Fe(s)+3O2(g)2Fe2O3(s)H=?arrow_forwardDetermine whether the statements given below are true or false. Consider an endothermic process taking place in a beaker at room temperature. (a) Heat flows from the surroundings to the system. (b) The beaker is cold to the touch. (c) The pressure of the system decreases. (d) The value of q for the system is positive.arrow_forward
- The temperature of the cooling water as it leaves the hot engine of an automobile is 240 F. After it passes through the radiator it has a temperature of 175 F. Calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a specific heat of 4.184 J/g oC.arrow_forwardConsider the following reaction in the vessel described in Question 57. A(g)+B(g)C(s)For this reaction, E=286 J, the piston moves up and the system absorbs 388 J of heat from its surroundings. (a) Is work done by the system? (b) How much work?arrow_forwardWhen 2.50 g of methane burns in oxygen, 125 kJ of heat is produced. What is the enthalpy of combustion per mole of methane under these conditions?arrow_forward
- Define heat. What are its units? How does it differ from energy?arrow_forwardWhat mass of acetylene, C2H2(g), must be burned to produce 3420 kJ of heat, given that its enthalpy of combustion is 1301 kJ/mol? Compare this with the answer to Exercise 5.91 and determine which substance produces more heat per gram.arrow_forwardA piece of chocolate cake contains about 400 calories. A nutritional calorie is equal to 1000 calories (thermochemical calories), which is equal to 4.184 kJ. How many 8-in-high steps must a 180-lb man climb to expend the 400 Cal from the piece of cake? See Exercise 28 for the formula for potential energy.arrow_forward
- Explain why absolute enthalpies and energies cannot be measured, and only changes can be determined.arrow_forwardIn a bomb calorimeter, the reaction vessel is surrounded by water that must be added for each experiment. Since the amount of water is not constant from experiment to experiment, the mass of water must be measured in each case. The heat capacity of the calorimeter is broken down into two parts: the water and the calorimeter components. If a calorimeter contains 1.00 kg water and has a total heat capacity of 10.84 kJ/C, what is the heat capacity of the calorimeter components?arrow_forwardIf 125 J of heat energy is applied to a block of silver weighing 29.3 g, by how many degrees will the temperature of the silver increase? (See Table 10.1.)arrow_forward
 World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning





