
Interpretation: The configuration and chirality should be identified for the given molecule by using its structure.
Concept Introduction:
Chiral carbon: Chiral atom is the one which is bonded to four different molecules or groups.
Configuration of a molecule: The configurations of a molecule arise due to the spatial arrangement of atoms. The configuration can be assigned by following CIP rules as follows.
Rules
Find the chiral carbon atom in a molecule.
Assign numbering to the groups bonded to the chiral carbon based on the molecular weight and electronegativity.
If the sequence of the numbering follows clockwise direction the chiral atom is assigned as R configuration.
If the sequence of the numbering follows anticlockwise direction the chiral atom is assigned as S configuration
The R and S configuration of a molecule can be interchanged when a least prior group present in above plane.
To find: The chiral atoms present of the given molecule.
Answer to Problem 31PP
The R and S nomenclature for the given molecule are shown below.
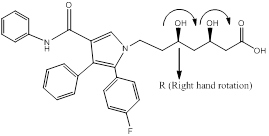
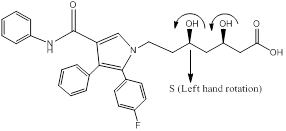
Explanation of Solution
- Draw the structure of the given molecule and find the chiral centers.
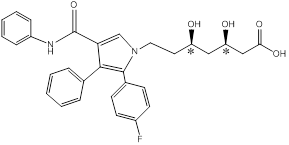
Chiral centers of the molecule are indicated by using *
The chiral center in the given molecule is marked using*. The * Carbons are chiral since all the carbons are
The presence of an asymmetric carbon center is one of several structural features that induce chirality in organic molecules.
- Assign absolute configuration R-S for the given molecules using CIP rules.
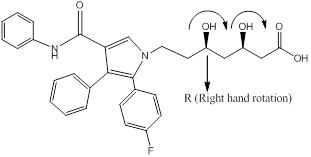
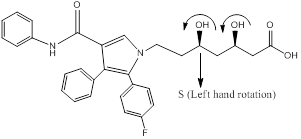
Chiral carbons are labeled as shown above molecules. Further the right and left hand nomenclature is used to name the enantiomers of chiral compounds. The stereocenter are indicated as R or S.
From the priority is assigned of the above molecules, according to the substitution of elements with higher
Want to see more full solutions like this?
Chapter 5 Solutions
ORGANIC CHEMISTRY..LL W/SM, SG,SM ACCES
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





