
Interpretation:
It is to be explained that the
Concept introduction:
The reaction of methyl and primary alcohols with hydrogen halide proceeds through
The
The reaction of tertiary alcohol with hydrogen halide proceeds through
The
The slow step in the
The carbocation (positively charged carbon) is
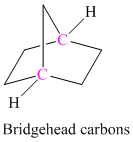
The carbocation at bridgehead carbon is unstable.
The carbon atom which is a part of two or more rings in polycyclic compounds is as shown below:
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Organic Chemistry - Standalone book
- In 1935, J. Bredt, a German chemist, proposed that a bicycloalkene could not have a double bond at a bridgehead carbon unless one of the rings containsat least eight carbons. This is known as Bredt’s rule. Explain why there cannot be a double bond at this position.arrow_forwardFollowing are the alternative chair conformations for trans-1,2-dimethylcyclohexane.(a) Estimate the difference in free energy between these two conformations.(b) Given your value in (a), calculate the percent of each chair present in anequilibrium mixture of the two at 25°C.arrow_forward1. Estimate the relative stabilities of the 4 conformationseclipsed and eclipsed n-pentane by drawing the corresponding Newman projections in descending order according to their stability. Place the most stable or lowest energy on the left. 2. Explain what the difference in energy between the most stable and the least stable is due to. 3. What is the difference in stability between the two staggered conformations?arrow_forward
- In some nucleophilic substitutions under SN1 conditions, complete racemization does not occur and a small excess of one enantiomer is present. For example, treatment of optically pure 1-bromo-1-phenylpropane with water forms 1- phenylpropan-1-ol. (a) Calculate how much of each enantiomer is present using the given optical rotation data. (b) Whichproduct predominates—the product of inversion or the product of retention of conguration? (c) Suggest an explanation for this phenomenon.arrow_forwardGiven that the change in free energy between axial and equatorial chlorine is 0.52 kcal.mol-1 , what is the difference in energy between the two chair conformations of 1,2,3,5-tetrachlorocyclohexane?What is the percent of the most stable chair conformation in solution at 298K?arrow_forwardStretch the structure for (S)-3-bromo-2-methylpentane and add the stereochemistry at all stereogenic centers.arrow_forward
- Rank the following groups in order of decreasing priority according to the Cahn-Ingold-Prelog system. I) -CH=CH2 II) -CH3 III) -CH IV) -OH 1) IV > III > I > II 2) IV > I > III > II 3) III > IV > I > II 4) II > I > III > IVarrow_forwardIn some nucleophilic substitutions under SN1 conditions, complete racemization does not occur and a small excess of one enantiomer ispresent. For example, treatment of optically pure 1-bromo-1phenylpropane with water forms 1-phenylpropan-1-ol. (a) Calculate how much of each enantiomer is present using the given optical rotation data. (b) Which product predominates—the product of inversion or the product of retention of configuration? (c) Suggest an explanation for this phenomenon.arrow_forwardGibbs free energy differences between axial-substituted and equatorial-substituted chair conformations of cyclohexane were given in Table 2.4. (a) Calculate the ratio of equatorial to axial tert-butylcyclohexane at 25C. (b) Explain why the conformational equilibria for methyl, ethyl, and isopropyl substituents are comparable but the conformational equilibrium for tert-butylcyclohexane lies considerably farther toward the equatorial conformation.arrow_forward
- Explain why compound A is much more stable than compound B.arrow_forwardWe saw that the energy cost of an axial methyl is 1.8 kcal/mol; therefore, we might expect cis-1,3-dimethylcyclohexane to have chair conformations with a difference in totalenergy of 3.6 kcal/mol. 1. Is the calculated energy difference in cis-1,3-dimethylcyclohexane higher or lower than expected? Propose an explanation for the difference between the expected energy difference and the calculated energy difference in cis-1,3-dimethylcyclohexane. (Limit your answer to 10 words or fewer) 2. Recall that an axial methyl is typically worth around 1.8 kcal/mol. Propose an explanation for the difference in the value above for 1-methyltetrahydropyran. (Limit your answer to 10 words or less) 3. Would you expect the difference in strain energy between the chairs of 5-methyl-1,3-dioxane to be greater than or less than that of 1-methyltetrahydropyran? Is it GREATER THAN or LESS THAN?arrow_forwardWrite structures for products A, B, C, and D showinf stereochemistry. (Hint: B and D are stereoisomers)arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
