
(1)
The phases present in chemical composition.
(1)
Explanation of Solution
Introduction:
A phaseis a homogeneous portion of a material that has uniform physical and chemical characteristics. A phase transformation occurs when a material changes from one phase to another. Phase transformations enter into many material processes, such as when parts are soldered or welded, or when metal is melted and cast into a shape that is then cooled to form a solid part.
(a)
The composition of
(b)
The composition of
(c)
The composition of
(d)
The composition of
(e)
The composition of
Tabulate the obtained result.
| S.No | Atomic Percent Nickel | Temperature | Phase |
| 1 | |||
| 2 | |||
| 3 | |||
| 4 | |||
| 5 |
Table (1)
Conclusion:
Therefore, the phases present in chemical composition is shown in Table (1).
(2)
The degree of freedom in the material from Gibbs phase rule.
(2)
Answer to Problem 5.13P
The degree of freedom is shown in Table (2) .
Explanation of Solution
Formula Used:
Write the expression for the Gibbs phase rule as:
Here,
Calculation:
(a)
Substitute
(b)
Substitute
(c)
Substitute
(d)
Substitute
(e)
Substitute
Tabulate the obtained result.
| S.No | Atomic Percent Nickel | Temperature | Degree of freedom |
| 1 | |||
| 2 | |||
| 3 | |||
| 4 | |||
| 5 |
Table (2)
Conclusion:
Therefore, the degree of freedom is shown in Table (2) .
(3)
The chemical composition of phase.
(3)
Explanation of Solution
Introduction:
Many two-component materials have two phases in equilibrium that have different chemical compositions. Low-carbon steel has the two components iron and carbon, and at room temperature it has two phases. The first phase is
iron carbide (Fe3C).
(a)
The chemical composition present at the temperature of
(b)
The chemical composition present at the temperature of
Thus,
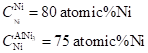
(c)
The chemical composition present at the temperature of
Thus,
(d)
The chemical composition present at the temperature of
Thus,
(e)
The chemical composition present at the temperature of
Thus,
Tabulate the obtained result.
| S.No | Atomic Percent Nickel | Temperature | Chemical composition |
| 1 | |||
| 2 | 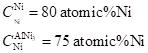 | ||
| 3 |
| ||
| 4 |
| ||
| 5 |
|
Table (3)
Conclusion:
Thus, the chemical composition of phase is shown in Table (3).
(4)
The atomic fraction of each phase.
(4)
Answer to Problem 5.13P
The atomic fraction for each phase is shown in Table (4) .
Explanation of Solution
Formula Used:
For
Write the expression for the atomic fraction of the
Here,
For
Write the expression for the atomic fraction of the
For
Write the expression for the atomic fraction of the
Here,
For
Write the expression for the atomic fraction of the
Calculation:
(a)
The atomic fraction of the Ni phase at the temperature of
(b)
Substitute
Substitute
(c)
Substitute
Substitute
(d)
The Peritectic reaction occurs at the temperature of
(e)
The atomic fraction of
Tabulate the obtained result.
| S.No | Atomic Percent Nickel | Temperature | Atomic fraction |
| 1 | |||
| 2 | |||
| 3 | |||
| 4 | Not possible | ||
| 5 |
Table (4)
Conclusion:
Thus, the atomic fraction for each phase is shown in Table (4) .
Want to see more full solutions like this?
Chapter 5 Solutions
Materials Science And Engineering Properties
 Materials Science And Engineering PropertiesCivil EngineeringISBN:9781111988609Author:Charles GilmorePublisher:Cengage Learning
Materials Science And Engineering PropertiesCivil EngineeringISBN:9781111988609Author:Charles GilmorePublisher:Cengage Learning
