
Concept explainers
5-16 Answer true or false.
(a) For a sample of gas at constant temperature, its pressure multiplied by its volume is a constant.
(b) For a sample of gas at constant temperature, increasing the pressure increases the volume.
(c) For a sample of gas at constant temperature, 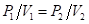
(d) As a gas expands at constant temperature, its volume increases.
(e) The volume of a sample of gas at constant pressure is directly proportional to its temperature—the higher its temperature, the greater its volume.
(f) A hot-air balloon rises because hot air is less dense than cooler air.
(g) For a gas sample in a container of fixed volume, an increase in temperature results in an increase in pressure.
(h) For a gas sample in a container of fixed volume, 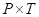 is a constant.
is a constant.
(i) When steam at 100°C in an autoclave is heated to 1200C, the pressure within the autoclave increases.
(j) When a gas sample in a flexible container at constant pressure at 25°C is heated to 50°C, its volume doubles.
(k) Lowering the diaphragm causes the chest cavity to increase in volume and the pressure of air in the lungs to decrease.
(l) Raising the diaphragm decreases the volume of the chest cavity and forces air out of the lungs.
(a)
Interpretation:
Find if the given statement is true or false.
“For a sample of gas at constant temperature, its pressure multiplied by its volume is a constant.”
Concept Introduction:
According to Boyle’s Law, the volume of fixed amount of gas is inversely proportional to the pressure of the gas at constant temperature. Mathematically, it is given as.
Answer to Problem 5.16P
For a sample of gas at constant temperature, its pressure multiplied by its volume is a constant, the given statement is true.
Explanation of Solution
Given information:
For a sample of gas at constant temperature, its pressure multiplied by its volume is a constant.
We know, according to Boyle’s Law, we have.
Thus, for a sample of gas at constant temperature, its pressure multiplied by its volume is a constant.
(b)
Interpretation:
Find if the given statement is true or false.
“For a sample of gas at constant temperature, increasing the pressure increases the volume.”
Concept Introduction:
According to Boyle’s Law, the volume of fixed amount of gas is inversely proportional to the pressure of the gas at constant temperature. Mathematically, it is given as.
Answer to Problem 5.16P
For a sample of gas at constant temperature, increasing the pressure decreases the volume thus, the given statement is false.
Explanation of Solution
Given Information:
For a sample of gas at constant temperature, increasing the pressure increases the volume.
We know, according to Boyle’s Law, we have.
That means there is an inverse relationship between volume and pressure at constant temperature. When pressure is increased, volume is decreased and vice versa.
Thus, for a sample of gas at constant temperature, increasing the pressure decreases the volume.
(c)
Interpretation:
Find if the given statement is true or false.
“For a sample of gas at constant temperature,
Concept Introduction:
According to Boyle’s Law, the volume of fixed amount of gas is inversely proportional to the pressure of the gas at constant temperature. Mathematically, it is given as.
Answer to Problem 5.16P
For a sample of gas at constant temperature,
Explanation of Solution
Given Information:
For a sample of gas at constant temperature,
We know, according to Boyle’s Law, we have.
We two different sets of volume and pressure of the gas is considered, the above equation becomes as follows:
where
Thus, for a sample of gas at constant temperature,
(d)
Interpretation:
Find if the given statement is true or false.
“As a gas expands at constant temperature, its volume increases.”
Concept Introduction:
According to Boyle’s Law, the volume of fixed amount of gas is inversely proportional to the pressure of the gas at constant temperature. Mathematically, it is given as.
Answer to Problem 5.16P
As a gas expands at constant temperature, its volume increases. Thus, the given statement is true.
Explanation of Solution
Given Information:
As a gas expands at constant temperature, its volume increases.
When a gas expands, the distance between the gas particles increases. Thus, pressure decreases.
Also, we know, according to Boyle’s Law.
Decrease in pressure increases the volume.
Thus, as a gas expands at constant temperature, its volume increases.
(e)
Interpretation:
Find if the given statement is true or false.
“The volume of a gas at constant pressure is directly proportional to its temperature − the higher its temperature, the greater its volume.”
Concept Introduction:
According to Charles’s Law, for the gas held at constant pressure, the volume of gas is directly proportional to the temperature of the gas. Mathematically, it is given as.
Answer to Problem 5.16P
The volume of a gas at constant pressure is directly proportional to its temperature − the higher its temperature, the greater its volume. Thus, the given statement is true.
Explanation of Solution
Given Information:
The volume of a gas at constant pressure is directly proportional to its temperature − the higher its temperature, the greater its volume.
We know, according to Charles’s Law, we have.
There is a direct relationship between volume and temperature. As the temperature is increased, the volume is also increased.
Thus, the volume of a gas at constant pressure is directly proportional to its temperature − the higher its temperature, the greater its volume.
(f)
Interpretation:
Find if the given statement is true or false.
“A hot-air balloon rises because hot air is less dense than cooler air”.
Concept Introduction:
Hot air rises because when the air is heated, it undergoes expansion. This results in the air to become less dense than the air surrounding it.
Answer to Problem 5.16P
A hot-air balloon rises because hot air is less dense than cooler air, thus, the given statement is true.
Explanation of Solution
Given information:
A hot-air balloon rises because hot air is less dense than cooler air.
Hot air rises because when the air is heated, it undergoes expansion. This results in the air to become less dense than the air surrounding it.
Thus, a hot-air balloon rises because hot air is less dense than cooler air.
(g)
Interpretation:
Find if the given statement is true or false.
“For a gas sample in a container of fixed volume, an increase in temperature results in the increase in pressure.”
Concept Introduction:
According to Gay-Lussac’s Law, for the gas held at constant volume, the pressure of a given amount of gas is directly proportional to the temperature of the gas. Mathematically, it is given as.
Answer to Problem 5.16P
For a gas sample in a container of fixed volume, an increase in temperature results in the increase in pressure. Thus, the given statement is true.
Explanation of Solution
Given information:
For a gas sample in a container of fixed volume, an increase in temperature results in the increase in pressure.
We know, according to Gay Lussac’s Law, we have.
There is a direct relationship between pressure and temperature. As the temperature is increased, the pressure is also increased.
Thus, for a gas sample in a container of fixed volume, an increase in temperature results in the increase in pressure.
(h)
Interpretation:
Find if the given statement is true or false.
“For a gas sample in a container of fixed volume,
Concept Introduction:
According to Gay-Lussac’s Law, for the gas held at constant volume, the pressure of a given amount of gas is directly proportional to the temperature of the gas. Mathematically, it is given as.
Answer to Problem 5.16P
For a gas sample in a container of fixed volume,
Explanation of Solution
Given information:
For a gas sample in a container of fixed volume, an increase in temperature results in the increase in pressure.
We know, according to Gay Lussac’s Law, we have.
Thus, for a gas sample in a container of fixed volume,
(i)
Interpretation:
Find if the given statement is true or false.
“When steam at
Concept Introduction:
According to Gay-Lussac’s Law, for the gas held at constant volume, the pressure of a given amount of gas is directly proportional to the temperature of the gas. Mathematically, it is given as.
Answer to Problem 5.16P
When steam at
Explanation of Solution
Given Information:
When steam at
We know, according to Gay Lussac’s Law, we have.
There is a direct relationship between pressure and temperature. As the temperature is increased, the pressure is also increased.
Thus, when steam at
(j)
Interpretation:
Find if the given statement is true or false.
“When a gas sample in a flexible container at constant pressure at
Concept Introduction:
According to Charles’s Law, for the gas held at constant pressure, the volume of gas is directly proportional to the temperature of the gas. Mathematically, it is given as.
Answer to Problem 5.16P
When a gas sample in a flexible container at constant pressure at
Explanation of Solution
Given Information:
When a gas sample in a flexible container at constant pressure at
We know, according to Charles’s Law, we have.
There is a direct relationship between volume and temperature. As the temperature is increased, the volume is also increased.
Now, the temperature of the container is doubled, hence the volume of the flexible container is also doubled.
Thus, when a gas sample in a flexible container at constant pressure at
(k)
Interpretation:
Find if the given statement is true or false.
“Lowering the diaphragm causes the chest cavity to increase in volume and the pressure of air in the lungs to decrease”.
Concept Introduction:
According to Boyle’s Law, the volume of fixed amount of gas is inversely proportional to the pressure of the gas at constant temperature. Mathematically, it is given as.
Answer to Problem 5.16P
Lowering the diaphragm causes the chest cavity to increase in volume and the pressure of air in the lungs to decrease thus, the given statement is true.
Explanation of Solution
Given information:
Lowering the diaphragm causes the chest cavity to increase in volume and the pressure of air in the lungs to decrease.
We know, according to Boyle’s Law, we have.
Now, when diaphragm is lowered, the chest cavity is increased. This results in the increase in volume and hence pressure decreases inside the lungs.
(l)
Interpretation:
Find if the given statement is true or false.
“Raising the diaphragm decreases the volume of the chest cavity and forces air out of the lungs.”
Concept Introduction:
According to Boyle’s Law, the volume of fixed amount of gas is inversely proportional to the pressure of the gas at constant temperature. Mathematically, it is given as.
Answer to Problem 5.16P
Raising the diaphragm decreases the volume of the chest cavity and forces air out of the lungs. Thus, the given statement is true.
Explanation of Solution
Given information:
Raising the diaphragm decreases the volume of the chest cavity and forces air out of the lungs.
We know, according to Boyle’s Law, we have.
Now, when diaphragm is raised, the volume chest cavity is decreased. This results in the decrease in volume and hence pressure increased inside the lungs and air is moved out of the lungs.
Want to see more full solutions like this?
Chapter 5 Solutions
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
- 5-37 A sample of a gas at 77°C and 1.33 atm occupies a volume of 50.3 L. (a) How many moles of the gas are present? (b) Does your answer depend on knowing what gas it is?arrow_forward5-107 If 60.0 g of NH3 occupies 35.1 L under a pressure of 77.2 in. Hg, what is the temperature of the gas, in °C?arrow_forward5-34 A sample of 30.0 mL of krypton gas, Kr, is at 756 mm Hg and 25.0°C. What is the new volume if the pressure is decreased to 325 mm Hg and the temperature is decreased to-12.5°C?arrow_forward
- 5-27 A sample of the inhalation anesthetic gas Halothane, C2HBrCIF3, in a 500-mL cylinder has a pressure of 2.3 atm at 0°C. What will be the pressure of the gas if its temperature is warmed to 37 °C (body temperature)?arrow_forward5-114 Carbon dioxide gas, saturated with water vapor, can be produced by the addition of aqueous acid to calcium carbonate based on the following balanced net ionic equation: (a) How many moles of wet CO (g), collected at 60.°C and 774 torr total pressure, are produced by the complete reaction of 10.0 g of CaCO3 with excess acid? (b) What volume does this wet CO2 occupy? (c) What volume would the CO2 occupy at 774 torr if a desiccant (a chemical drying agent) were added to remove the water? The vapor pressure of water at 60.°C is 149.4 mm Hg.arrow_forward5-111 Diving, particularly SCUBA (Self-Contained Underwater Breathing Apparatus) diving, subjects the body to increased pressure. Each 10. m (approximately 33 ft) of water exerts an additional pressure of 1 atm on the body. (a) What is the pressure on the body at a depth of 100. ft? (b) The partial pressure of nitrogen gas in air at 1 atm is 593 mm Hg. Assuming a SCUBA diver breathes compressed air, what is the partial pressure of nitrogen entering the lungs from a breathing tank at a depth of 100. ft? (c) The partial pressure of oxygen gas in the air at 2 atm is 158 mm Hg. What is the partial pressure of oxygen in the air in the lungs at a depth of 100. ft? (d) Why is it absolutely essential to exhale vigorously in a rapid ascent from a depth of 100. ft?arrow_forward
- 5-25 A gas in a bulb as in Figure 5-3 registers a pressure of 833 mm Hg in the manometer in which the reference arm of the U-shaped tube (A) is sealed and evacuated. What will the difference in the mercury levels be if the reference arm of the U-shaped tube is open to atmospheric pressure (760 mm Hg)?arrow_forward5-41 Does the density of a gas increase, decrease, or stay the same as the pressure increases at constant temperature? As the temperature increases at constant pressure?arrow_forward5-46 Calculate the molar mass of a gas if 3.30 g of the gas occupies 660. mL. at 735 mm Hg and 27°C.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
