
Concept explainers
(a)
Interpretation:
The products obtained from the reaction of
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Answer to Problem 5.27AP
The products obtained from the reaction of
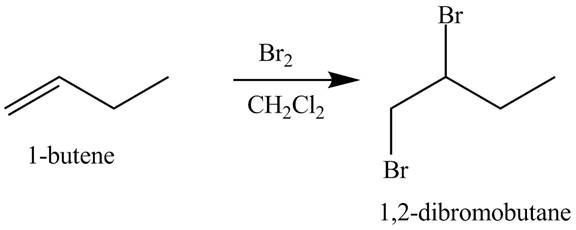
Explanation of Solution
The reaction of
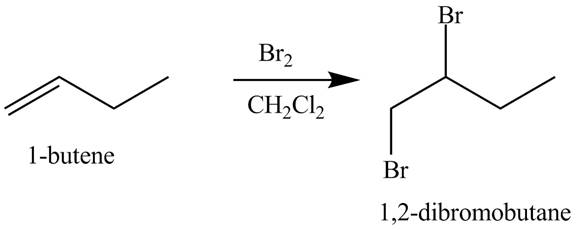
Figure 1
The products obtained from the reaction of
(b)
Interpretation:
The products obtained from the reaction of
Concept introduction:
Alkenes are the unsaturated class of organic compounds which have a double bond in their structure. The general formula of alkene is written as
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Answer to Problem 5.27AP
The products obtained from the reaction of
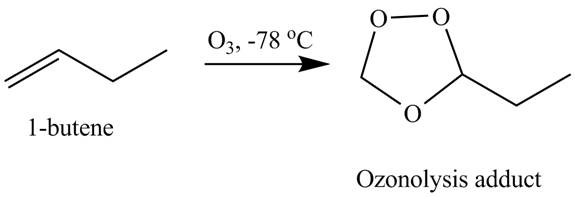
Explanation of Solution
The reaction of
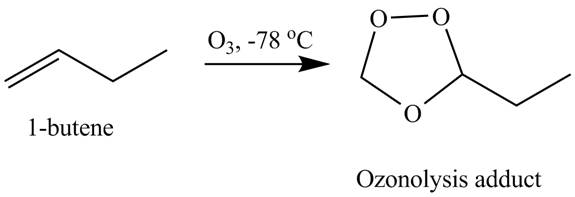
Figure 2
The products obtained from the reaction of
(c)
Interpretation:
The products obtained from the reaction of
Concept introduction:
Alkenes are the unsaturated class of organic compounds which have a double bond in their structure. The general formula of alkene is written as
The systematic naming of organic compound is given by
Answer to Problem 5.27AP
The products obtained from the reaction of the product of (b) with
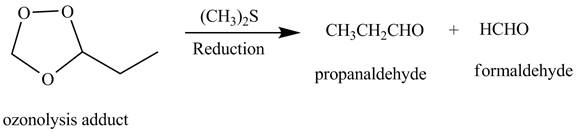
Explanation of Solution
The reaction of the product (b) that is ozonolysis adduct with
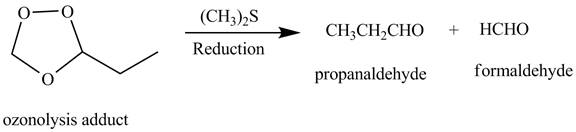
Figure 3
The products obtained from the reaction of the product of (b) with
(d)
Interpretation:
The products obtained from the reaction of
Concept introduction:
Alkenes are the unsaturated class of organic compounds which have a double bond in their structure. The general formula of alkene is written as
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Answer to Problem 5.27AP
The products obtained from the reaction of the product of (b) with
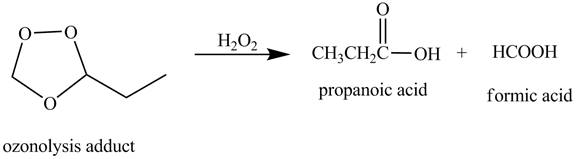
Explanation of Solution
The reaction of the product of part (b) with

Figure 4
The products obtained from the reaction of the product of (b) with
(e)
Interpretation:
The products obtained from the reaction of
Concept introduction:
Alkenes are the unsaturated class of organic compounds which have a double bond in their structure. The general formula of alkene is written as
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Answer to Problem 5.27AP
The products obtained from the reaction of
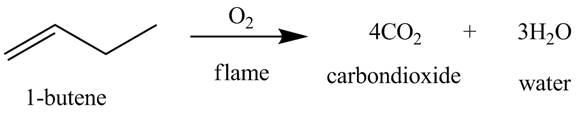
Explanation of Solution
The reaction of
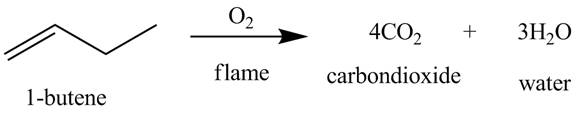
Figure 5
The products obtained from the reaction of
(f)
Interpretation:
The products obtained from the reaction of
Concept introduction:
Alkenes are the unsaturated class of organic compounds which have a double bond in their structure. The general formula of alkene is written as
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Answer to Problem 5.27AP
The products obtained from the reaction of
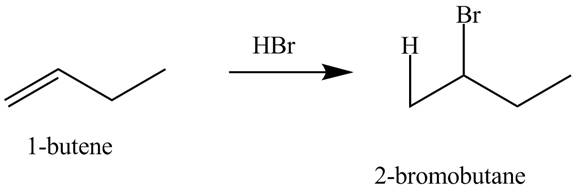
Explanation of Solution
The reaction of

Figure 6
The products obtained from the reaction of
(g)
Interpretation:
The products obtained from the reaction of
Concept introduction:
Alkenes are the unsaturated class of organic compounds which have a double bond in their structure. The general formula of alkene is written as
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Answer to Problem 5.27AP
The products obtained from the reaction of
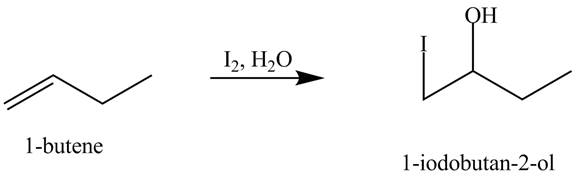
Explanation of Solution
The reaction of

Figure 7
The products obtained from the reaction of
(h)
Interpretation:
The products obtained from the reaction of
Concept introduction:
Alkenes are the unsaturated class of organic compounds which have a double bond in their structure. The general formula of alkene is written as
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Answer to Problem 5.27AP
The products obtained from the reaction of
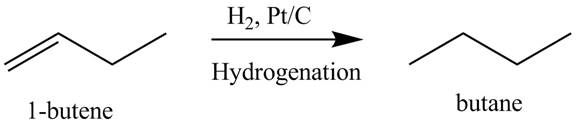
Explanation of Solution
The reaction

Figure 8
The products obtained from the reaction of
(i)
Interpretation:
The products obtained from the reaction of
Concept introduction:
Alkenes are the unsaturated class of organic compounds which have a double bond in their structure. The general formula of alkene is written as
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Answer to Problem 5.27AP
The products obtained from the reaction of
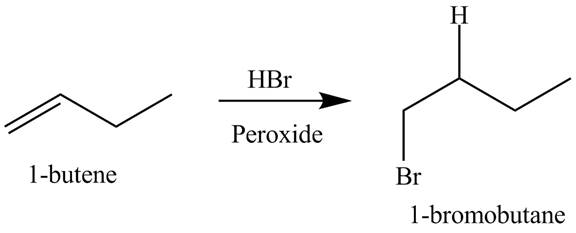
Explanation of Solution
The reaction of

Figure 9
The products obtained from the reaction of
(j)
Interpretation:
The products obtained from the reaction of
Concept introduction:
Alkenes are the unsaturated class of organic compounds which have a double bond in their structure. The general formula of alkene is written as
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Answer to Problem 5.27AP
The products obtained from the reaction of
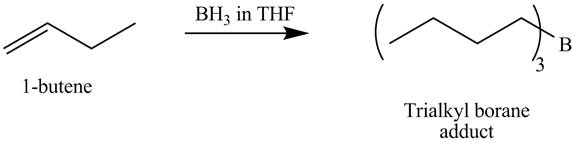
Explanation of Solution
The reaction of

Figure 10
The products obtained from the reaction of
(k)
Interpretation:
The products obtained from the reaction of the product of part (j) and
Concept introduction:
Alkenes are the unsaturated class of organic compounds which have a double bond in their structure. The general formula of alkene is written as
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Answer to Problem 5.27AP
The products obtained from the reaction of the product of part (j) and
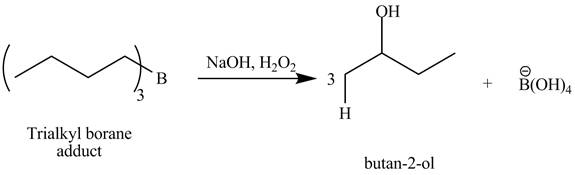
Explanation of Solution
The reaction of a trialkyl borane adduct that is obtained from part (j) with

Figure 11
The products obtained from the reaction of the product of part (j) and
(l)
Interpretation:
The products obtained from the reaction of
Concept introduction:
Alkenes are the unsaturated class of organic compounds which have a double bond in their structure. The general formula of alkene is written as
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Answer to Problem 5.27AP
The products obtained from the reaction of
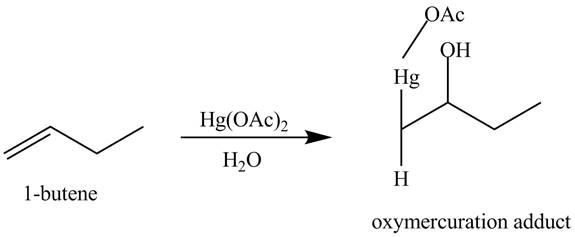
Explanation of Solution
The reaction of
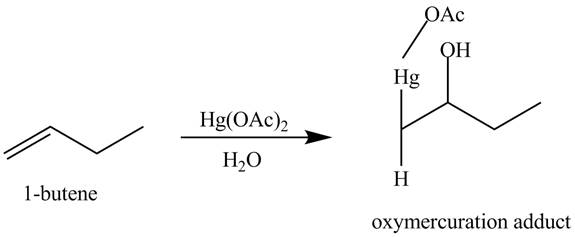
Figure 12
The products obtained from the reaction of
(m)
Interpretation:
The products obtained from the reaction of the product of part (l) and
Concept introduction:
Alkenes are the unsaturated class of organic compounds which have a double bond in their structure. The general formula of alkene is written as
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Answer to Problem 5.27AP
The products obtained from the reaction of the product of part (l) and
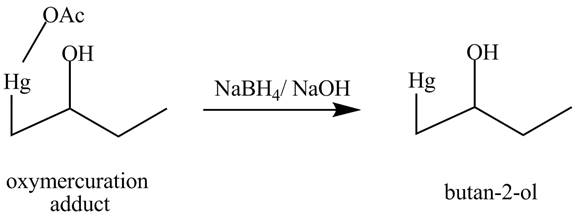
Explanation of Solution
The reaction of the product of part (l) reaction with

Figure 13
The products obtained from the reaction of the product of part (l) and
(n)
Interpretation:
The products obtained from the reaction of
Concept introduction:
Alkenes are the unsaturated class of organic compounds which have a double bond in their structure. The general formula of alkene is written as
Answer to Problem 5.27AP
The products obtained from the reaction of
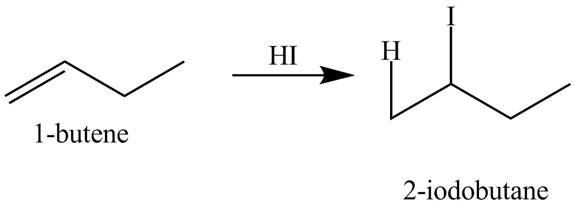
Explanation of Solution
The reaction of

Figure 14
The products obtained from the reaction of
(o)
Interpretation:
The products obtained from the reaction of
Concept introduction:
Alkenes are the unsaturated class of organic compounds which have a double bond in their structure. The general formula of alkene is written as
Answer to Problem 5.27AP
The products obtained from the reaction of
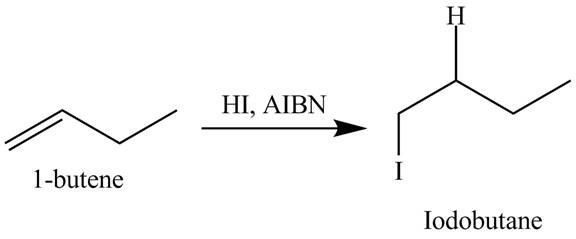
Explanation of Solution
The reaction of

Figure 15
The products obtained from the reaction of
Want to see more full solutions like this?
Chapter 5 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- 3b)Give the mechanisms for the following transformations:arrow_forwardAmines are converted into alkenes by a two-step process called Hofmann elimination. SN2 reaction of the amine with an excess of CH3I in the first step yields an intermediate that undergoes E2 reaction when treated with silver oxide as base. Pentylamine, for example, yields 1-pentene. Propose a structure for the intermediate, and explain why it readily undergoes elimination.arrow_forwardHydrocarbon X has the formula C6H12.X reacts with one molar equivalent of hydrogen in the presence of a palladium catalyst to form a product having 12 primary hydrogens.Treatment of X with ozone followed by zinc in aqueous acid gives a mixture two aldehydes.What is the structure of X?arrow_forward
- 4. Compound A has the formula C 8H 8. It reacts rapidly with KMnO 4 to give CO 2 and a carboxylic acid, B (C 7H 6O 2), but reacts with only 1 molar equivalent of H 2 on catalytic hydrogenation over a palladium catalyst. On hydrogenation under conditions that reduce aromatic rings, 4, equivalents of H 2 are taken up and hydrocarbon C (C 8H 16) is produced. What are the structures of A, B, and C.arrow_forwardStarting with acetylene and ethylene oxide as the only sources of carbon atoms, show how to prepare the compound Q.)1,6-Hexanediolarrow_forwardIsoprene has sometimes been used as a starting material in the laboratory synthesis of terpenes. In one such synthesis, the first step is the electrophilic addition of 2 mol of hydrogen bromide to isoprene to give 1,3-dibromo-3-methylbutane.Write a series of equations describing the mechanism of this reaction.arrow_forward
- Starting with acetylene and ethylene oxide as the only sources of carbon atoms, show how to prepare the compound Q.)Hexanedialarrow_forwardAn organic compound A of unknown structure was found to have a molecular formula C8H16. When A was poured in water and heated, compound B having a molecular formula C8H18O was formed. B upon heating with sulfuric acid was converted to C as the major product which is identical to A. Ozonolysis of C gave one molecule each of two different products D and E, both having a molecular formula C4H8O. Write the reactions involved and determine the structure of A,B,C,D and E.arrow_forwardProvide the reagents necessary to convert 3-methyl-2-butanol to 2-bromo-3-methylbutane. CHOOSE FROM THE FOLLOWING REAGRENTS BELOW conc. HBr NaBr, H2SO4 PBr3 HBr, peroxide Br2arrow_forward
- 2. An unknown hydrocarbon A with the formula C 6H 12 reacts with 1 molar equivalent of H 2 over palladium catalyst. Hydrocarbon A also reacts with OsO 4 to give diol B. When oxidized with KMnO 4 in acidic solution, A gives two fragments. One fragment is propanoic acid, CH3CH 2CO 2H and the other fragment is a ketone. Which of the following are correctarrow_forwardCompound A (C6H12O2) reacts with water, acid, and heatto yield compound B (C5H10O2) and compound C (CH4O).Compound B is acidic. Deduce possible structures of compounds A, B, and Carrow_forwardCompound AA has a molecular formula of C3H6O and gives a positiveresult using Tollen’s reagent. The reaction of compound AA with hotacidified potassium permanganate, KMnO4 gives compound BB. Thecatalytic hydrogenation of compound AA with nickel, Ni producedcompound CC. The reaction of compound BB with ethanamine,CH3CH2NH2 produces compound DD I) Draw the structural formula of compounds AA, BB, CC and DD. 2)Name the type of chemical reaction for the formation of compound CC.arrow_forward

 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



