
Concept explainers
(a)
Interpretation:
Principal organic product obtained on reaction of
Concept introduction:
In an electrophilic addition reaction, there is an addition of an electrophilic reagent on a nucleophilic group. The double bond and triple bond containing compounds act as a nucleophile. The addition of electrophilic reagent on nucleophilic compound leads to the dissociation of
Answer to Problem 5.28AP
The product formed on reaction of
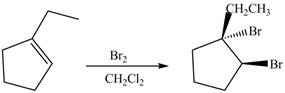
Explanation of Solution
In the reaction of
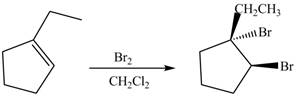
Figure 1
The product formed by reaction of
The reaction between
(b)
Interpretation:
Principal organic product obtained on reaction of
Concept introduction:
In the reactionof
Answer to Problem 5.28AP
The product formed by the reaction of
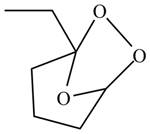
Explanation of Solution
In the reaction,
The general view of
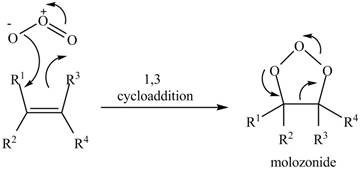
Figure 2
In the same manner,
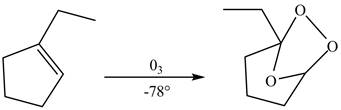
Figure 3
The product formed by the reaction between
(c)
Interpretation:
Principal organic product obtained on the reaction of
Concept introduction:
In the reactionof alkene with ozone, the oxidation of alkene takes place which leads to form molozonide product. This reaction takes place through
Answer to Problem 5.28AP
The product formed by the reaction of
Explanation of Solution
The compound
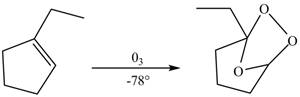
Figure 3
The molozonide formed is unstable in nature, therefore, it gets easily reduced by
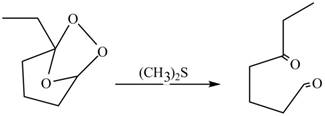
Figure 4
Therefore, the product formed by the reaction between
The reaction between
(d)
Interpretation:
Principal organic product obtained on the reaction of
Concept introduction:
In the reactionof alkene with ozone, the oxidation of alkene takes place which leads to form molozonide product. This reaction takes place through
Answer to Problem 5.28AP
The product formed by the reaction of
Explanation of Solution
The compound
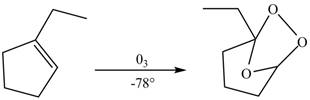
Figure 3
The molozonide formed is unstable in nature, therefore, it gets easily oxidized by
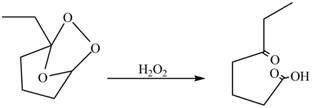
Figure 5
Therefore, the product formed by the reaction between
The reaction between
(e)
Interpretation:
Principal organic product on reaction of
Concept introduction:
The reaction between unsaturated alkene with
Answer to Problem 5.28AP
The product formed by the reaction of
Explanation of Solution
The reaction between
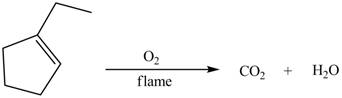
Figure 6
Therefore, the reaction between
The reaction between
(f)
Interpretation:
Principal organic product on reaction of
Concept introduction:
In markovnikov’s addition of
Answer to Problem 5.28AP
The product formed by the reaction of
Explanation of Solution
In the reaction of
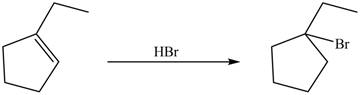
Figure 7
Therefore, the product formed by the reaction between
Thereaction between
(g)
Interpretation:
Principal organic product on reaction of
Concept introduction:
.In an electrophilic addition reaction, there is an addition of an electrophilic reagent on a nucleophilic group. The double bond and triple bond containing compounds act as nucleophile. The addition of electrophilic reagent on nucleophilic compound leads to the dissociation of
Answer to Problem 5.28AP
The product formed by the reaction of
Explanation of Solution
In the reaction first reaction between
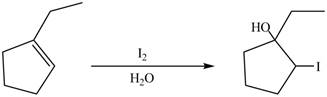
Figure 8
Therefore, the reaction between
The reaction between
(h)
Interpretation:
Principal organic product on reaction of
Concept introduction:
In acatalytic hydrogenation reaction, palladium is used as a catalyst which accelerates the
Answer to Problem 5.28AP
The product formed by the reaction of
Explanation of Solution
In this reaction, palladium is used as a catalyst which helps in accelerating the rate of hydrogenation reaction. This reaction leads to conversion of
The product formed by the reaction between
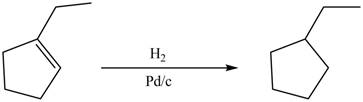
Figure 9
Theproduct formed by the reaction between
(i)
Interpretation:
Principal organic product on reaction of
Concept introduction:
In Anti-Markovnikov’s addition of
Answer to Problem 5.28AP
The product formed on reaction of
Explanation of Solution
In thereaction, in presence of peroxides,
The product formed by the reaction between
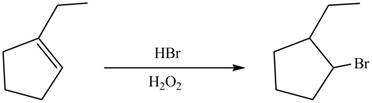
Figure 10
The product formed by the reaction between
(j)
Interpretation:
Principal organic product on reaction of ![]() with
with
Concept introduction:
In hydroboration reaction, antimarkovnikov’s addition of
Answer to Problem 5.28AP
The product formed by thereaction of
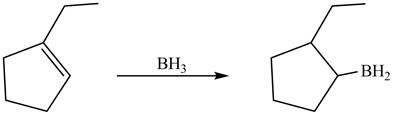
Explanation of Solution
The reaction between
The product formed by thereaction between
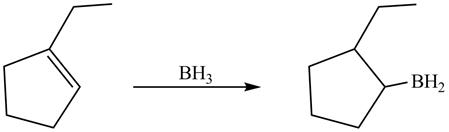
Figure 11
The product formed by the reaction between
(k)
Interpretation:
Principal organic product formed by the reaction of product of
Concept introduction:
In hydroboration reaction, antimarkovnikov’s addition of
The compound
Answer to Problem 5.28AP
The product formed on reaction of boron substitued
Explanation of Solution
In the reaction,.the hydroxide group of
The product formed by the reaction between boron substitued
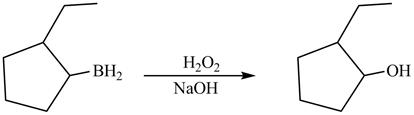
Figure 12
The product formed by thereaction between boron substitued
(l)
Interpretation:
Principal organic product on reaction of
Concept introduction:
Inan oxymercuration reaction,
Answer to Problem 5.28AP
The product formed on reaction of
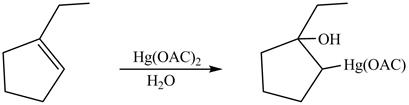
Explanation of Solution
This is an oxymercuration reaction in which
The product formed by the reaction between
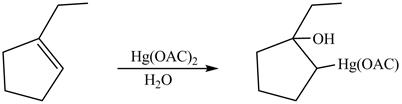
Figure 13
The product formed by thereaction between
(m)
Interpretation:
Principal organic product formed by the treatment of product of reaction of
Concept introduction:
In an oxymercuration reaction,
In demercuration reaction,
Answer to Problem 5.28AP
The Principal organic product formed by the treatment of product of reaction of
Explanation of Solution
The product formed by the reaction between
In demercuration reaction,
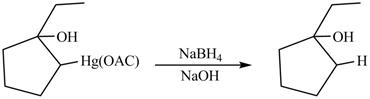
Figure 14
The product formed on reaction is
The product formed by the treatment of product of reaction of
(n)
Interpretation:
Principal organic product formed by the treatment of product of reaction of
Concept introduction:
In an electrophilic addition reaction, there is an addition of an electrophilic reagent on a nucleophilic group. The double bond and triple bond containing compounds act as a nucleophile. The addition of electrophilic reagent on nucleophilic compound leads to the dissociation of
Answer to Problem 5.28AP
The product formed by the treatment of product of reaction of
Explanation of Solution
The reaction between
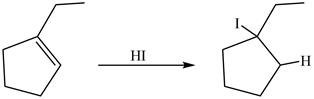
Figure 15
Principal organic product formed by the treatment of product of reaction of
(o)
Interpretation:
Principal organic product formed by the treatment of product of reaction of
Concept introduction:
The reaction between an alkene,
Answer to Problem 5.28AP
The Principal organic product formed by the treatment of product of reaction of
Explanation of Solution
The reaction between ![]() in presence of
in presence of
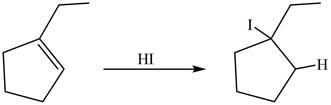
Figure 15
The product formed by the treatment of product of reaction of
Want to see more full solutions like this?
Chapter 5 Solutions
Organic Chemistry, Ebook And Single-course Homework Access
- Explain why butane is customarily drawn in the sawtooth conformation that it is.arrow_forwardIllustrating with equations, indicate how cyclopentene would be converted to tert-butoxycyclopentanearrow_forwardConsider 1-bromopropane, CH3CH2CH2Br. (a) Which of these is the lowest energy conformation?arrow_forward
- Explain briefly and clearly the following concepts, taking as reference the molecule of n-butane and the corresponding drawings or illustrations. See pages 149-152 of the book Organic Chemistry, sixth edition (J. G. Smith). 4. What is steric hindrance in a conformation? Then draw a picture to illustrate the concept? 5. What is the torsional stress of a conformation? Then draw a picture to illustrate the concept? 6. Describe 1,3-diaxial interaction and illustrate with a specific example.arrow_forwardAccording to the following structure, draw the Newmann projections in which you must indicate: a) the most stable conformation and explain why it is more stable, b) the least stable conformation and explain why; c) a conformation where there is a gauche interaction.arrow_forwardCalculate the destabilization present in each eclipsed conformation.arrow_forward
- For each newman projection, present in the conformational analysis of butane, draw a corresponding structure in sawhorse notation.arrow_forwardPlease write the answer clearly with the state of matter following each compound.arrow_forwardDetermine the element of unsaturation (aka unsaturation number) for the compounds or formulas belowarrow_forward
- Knowing that cyclohexane and hexene are isomers, suggest 2 methods allowing their separation.arrow_forwardProvide the least stable conformation of the following compound. Iarrow_forwardAmong the figure, (a) what is the most stable conformation of n-pentane? (b) gauche conformation of butane and (c) least stable conformation of butanearrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
