
Concept explainers
(a)
Interpretation:
Structural formula of lycopene has to be drawn showing the two sets of four isoprene units linked to form lycopene.
Concept Introduction:
Terpenes are made by joining five-carbon units, usually in a head to tail-fashion.
Isoprene unit:
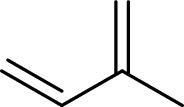
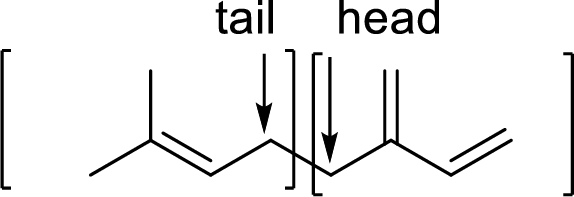
Branched end of isoprene – Head
Unbranched end of isoprene - Tail
(b)
Interpretation:
Number of double in lycopene that has a possibility of cis, trans isomerism has to be given and the double bond that shows cis and that shows trans isomers has to be identified.
Concept Introduction:
Terpenes are made by joining five-carbon units, usually in a head to tail-fashion.
Isoprene unit:
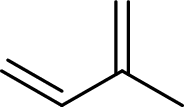
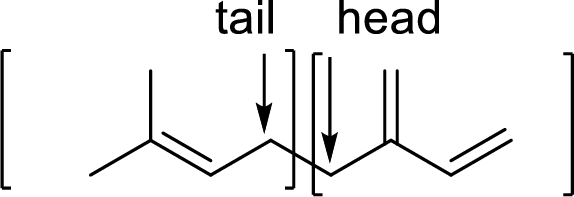
Branched end of isoprene – Head
Unbranched end of isoprene - Tail
Alkene:
- • Trans configuration: In trans configuration, carbon atoms present in the main chain are placed on opposite sides of the carbon-carbon double bond.
- • Cis configuration: In cis configuration, carbon atoms present in the main chain are placed on same sides of the carbon-carbon double bond.
Trending nowThis is a popular solution!

Chapter 5 Solutions
Organic Chemistry
- One of the principal components of lemongrass oil is limonene, C10H16. When limoneneis treated with excess hydrogen and a platinum catalyst, the product is an alkane offormula C10H20. What can you conclude about the structure of limonene?arrow_forward5. Compound A, C 10H 18O, undergoes reaction with dilute H 2SO 4 at 50 °C to yield a mixture of two alkenes, C 10H 16. The major alkene B, gives only cyclopentanone after ozone treatment followed by reduction with zinc in acetic acid. Which of the following reactions are correct.arrow_forwardWhat alkane, with molecular formula C7H16, forms seven monochlorinated products (disregarding stereoisomers) when heated with Cl2?arrow_forward
- Alcohol A (C10H18O) is converted to a mixture of alkenes B and C on being heated with potassium hydrogen sulfate (KHSO4). Catalytic hydrogenation of B and C yields the same product. Assuming that dehydration of alcohol A proceeds without rearrangement, deduce the structures of alcohol A and alkene C.arrow_forwardCompound AA has a molecular formula of C3H6O and gives a positiveresult using Tollen’s reagent. The reaction of compound AA with hotacidified potassium permanganate, KMnO4 gives compound BB. Thecatalytic hydrogenation of compound AA with nickel, Ni producedcompound CC. The reaction of compound BB with ethanamine,CH3CH2NH2 produces compound DD I) Draw the structural formula of compounds AA, BB, CC and DD. 2)Name the type of chemical reaction for the formation of compound CC.arrow_forwardPropose the structure of the following: a. An alkane, C6H14 b. A crylic saturated hydrocarbon, C6H12 c. A diene (dialkene), C5H8 d. A keto alkene, C5H8Oarrow_forward
- A compound with formula C7H12O is treated with sodium borohydride in methanol to yield 2,2-dimethylcylopentanol. Write a reaction scheme showing the structures of the reactant, the reagents, and the product. Will the product be optically active? Explain.arrow_forward2-bromopentane, when treated with alcoholic KOH yields a mixture of three alkenes A, B and C. Identify A, B and C. Which is predominant?arrow_forwardCompound A, C5H11Br, on treatment with alcoholic KOH, gives two isomeric compoundsarrow_forward
- Compounds X and Y are stereoisomers having the formula C6H12.Both X and Y react with one molar equivalent of hydrogen in the presence of a palladium catalyst to form hexane, and they each react with HBr to give a single bromoalkane product.Draw structural formulas for both X and Y.arrow_forwardFive isomeric alkanes (A–E) having the molecular formula C6H14 are each treated with Cl2 + hv to give alkyl halides having molecular formulaC6H13Cl. A yields five constitutional isomers. B yields four constitutionalisomers. C yields two constitutional isomers. D yields threeconstitutional isomers, two of which possess stereogenic centers. Eyields three constitutional isomers, only one of which possesses astereogenic center. Identify the structures of A–E.arrow_forwardAn unknown hydrocarbon A with the formula C6H12 reacts with 1 molar equivalent of H2 over a palladium catalyst. Hydrocarbon A also reacts with OsO4 to give diol B. When oxidized with KMnO4 in acidic solution, A gives two fragments. One fragment is propanoic acid, CH3CH2CO2H, and the other fragment is ketone C. What are the structures of A, B, and C? Write all reactions, and show your reasoning.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning



