
Concept explainers
(a)
Interpretation:
The number of chiral centers in the given molecule is to be identified and labeled with an asterisk.
Concept introduction:
A chiral center is a tetrahedral stereocenter. The atom at the chiral center must be
Answer to Problem 5.42P
There are four chiral centers in the given molecule marked with asterisk (*):
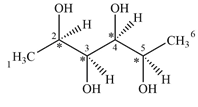
Explanation of Solution
Structure of the given molecule is:
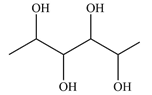
Expanding this line drawing to show the terminal carbons and adding hydrogen atoms at the remaining carbon atoms, the structure will look like
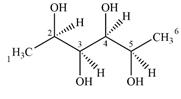
The terminal carbon atoms C1 and C6 are bonded to three hydrogen atoms. Therefore, they are not chiral centers.
C2 is bonded to the four groups,
C3 is bonded to the four groups
C4 is bonded to the four groups
C5 is bonded to the four groups
These four carbon atoms are bonded to four different groups and are chiral.
Therefore, there are four carbon atoms C2, C3, C4, and C5 are chiral centers
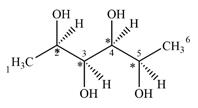
A carbon or a nitrogen atom is a chiral center if it is bonded to four different atoms or groups.
(b)
Interpretation:
The number of chiral centers in the given molecule is to be identified and labeled.
Concept introduction:
A chiral center is a tetrahedral stereocenter. The atom at the chiral center must be
Answer to Problem 5.42P
The given molecule has two chiral centers labeled with an asterisk as below:
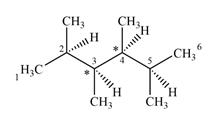
Explanation of Solution
The line drawing of the given molecule is
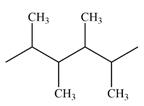
Expanding this line drawing to show the terminal carbons and adding hydrogen atoms at the remaining carbon atoms, the structure will look like
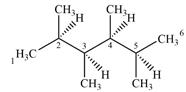
Both C1 and C6 are bonded to three hydrogens each. Therefore, they are not chiral centers.
C2 and C5 also are not chiral centers as two of the groups bonded to them are identical. Each one is bonded to two
C3 and C4 are bonded to four different groups
Therefore, these two carbon atoms are chiral centers
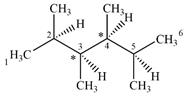
A carbon or a nitrogen atom is a chiral center if it is bonded to four different atoms or groups.
(c)
Interpretation:
The number of chiral centers in the given molecule is to be identified and labeled.
Concept introduction:
A chiral center is a tetrahedral stereocenter. The atom at the chiral center must be
Answer to Problem 5.42P
The given molecule has no chiral centers.
Explanation of Solution
The line drawing of the molecule is
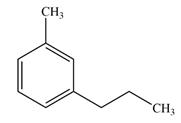
A chiral center must be an
In the alkyl substituent on the benzene ring, the terminal carbon has three hydrogen atoms bonded to it. The remaining two carbon atoms in the chain have two hydrogen atoms bonded to them. Therefore, these carbon atoms are also not chiral centers.
A carbon or a nitrogen atom is a chiral center if it is bonded to four different atoms or groups.
(d)
Interpretation:
The number of chiral centers in the given molecule is to be identified and labeled.
Concept introduction:
A chiral center is a tetrahedral stereocenter. The atom at the chiral center must be
Answer to Problem 5.42P
There is one chiral center in the given molecular ion:
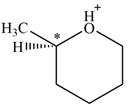
Explanation of Solution
Line drawing of the given molecular ion is
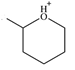
The molecule consists of a ring made up of five carbon atoms and one oxygen atom, with a methyl substituent on the carbon next to O. Oxygen does not form four bonds, and therefore, it cannot be a chiral center.
The carbon that has a methyl group bonded to it is also bonded to another (ring) carbon, the oxygen atom, and a hydrogen atom – all four are different. Therefore, this carbon is a chiral center. The remaining four ring carbons are bonded to two hydrogen atoms, so they cannot be chiral centers.
Therefore, there is only one chiral center in this molecular ion:
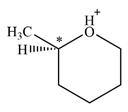
A carbon or a nitrogen atom is a chiral center if it is bonded to four different atoms or groups.
(e)
Interpretation:
The number of chiral centers in the given molecule is to be identified and labeled.
Concept introduction:
A chiral center is a tetrahedral stereocenter. The atom at the chiral center must be
Answer to Problem 5.42P
There is one chiral center C3 in the given molecular ion
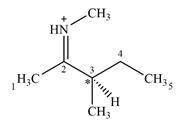
Explanation of Solution
The line drawing of the molecular ion is:
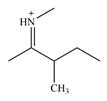
Adding in the terminal carbons as methyl groups and numbering the carbon chain as below helps in identifying which carbon atoms could be chiral centers:
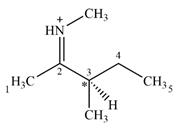
The four terminal carbon atoms are bonded to three hydrogen atoms each, and therefore cannot be chiral centers. C2 and the nitrogen atom are also not chiral centers since they are
C4 is also not a chiral center as it has two hydrogen atoms bonded to it.
C3 is the only carbon atom that is bonded to four different groups
Therefore, C3 is the only chiral center in the molecule.
A carbon or a nitrogen atom is a chiral center if it is bonded to four different atoms or groups.
(f)
Interpretation:
The number of chiral centers in the given molecule is to be identified and labeled.
Concept introduction:
A chiral center is a tetrahedral stereocenter. The atom at the chiral center must be
Answer to Problem 5.42P
There are two chiral centers in the given molecular ion:
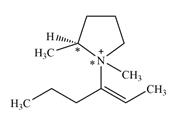
Explanation of Solution
The line drawing of the given molecular ion is
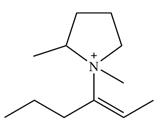
Adding in the terminal methyl groups gives the structure:
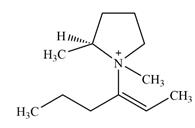
The terminal carbon atoms have three hydrogen atoms bonded to them, so they cannot be chiral centers. The two double bonded carbon atoms also cannot be chiral centers as chirality requires the atoms to be
The two remaining carbons in the alkenyl substituent on N have two hydrogen atoms bonded to each, and therefore cannot be chiral centers.
The ring carbon that is bonded to N is also bonded to three different groups,
The nitrogen atom is
Therefore, there are two chiral centers in this molecular ion:

A carbon or a nitrogen atom is a chiral center if it is bonded to four different atoms or groups.
(g)
Interpretation:
The number of chiral centers in the given molecule is to be identified and labeled.
Concept introduction:
A chiral center is a tetrahedral stereocenter. The atom at the chiral center must be
Answer to Problem 5.42P
There are no chiral centers in the given molecule.
Explanation of Solution
The line drawing of the molecule is:
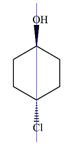
Four of the six ring carbons are bonded to two hydrogen atoms each. Therefore, they cannot be chiral centers.
The remaining two ring carbons have two identical groups bonded to them. The symmetry of the ring means the two sides are identical. These two carbons are also not chiral carbons.
Therefore, this molecule has no chiral centers.
A carbon or a nitrogen atom is a chiral center if it is bonded to four different atoms or groups.
Want to see more full solutions like this?
Chapter 5 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div





