
Concept explainers
The following figure shows three 1.00-L bulbs connected by valves. Each bulb contains argon gas with amounts proportional to the number of circles pictorially represented in the chamber. All three bulbs are maintained at the same temperature. Unless stated otherwise, assume that the valves connecting the bulbs are closed and seal the gases in their respective chambers. Assume also that the volume between each bulb is negligible.
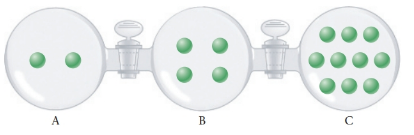
(a) Which bulb has the highest pressure?
(b) If the pressure in bulb A is 0.500 atm, what is the pressure in bulb C?
(c) If the pressure in bulb A is 0.500 atm, what is the total pressure?
(d) If the pressure in bulb A is 0.500 arm, and the valve between bulbs A and B is opened, redraw the figure shown above to accurately represent the gas atoms in all three bulbs. What is
(e) Follow the instructions of part (d) but now open only the valve between bulbs B and C.
(a)
Interpretation:
The bulb with the highest pressure needs to be identified based on the given description.
Concept introduction:
The ideal gas equation is a thermodynamic equation of state which relates the pressure (P), volume (V), number of moles (n) and temperature (T) of an ideal gas through the following expression:
where R is the universal gas constant = 0.0821 L.atm/mol-K
Answer to Problem 86QAP
Bulb C has the highest pressure.
Explanation of Solution
Given Information:
Volume (V) of bulb A = B = C = 1.0 L
Temperature (T) of bulb A = B = C
Number of moles (n) of Ar gas in A = 2
Number of moles (n) of Ar gas in B = 4
Number of moles (n) of Ar gas in C = 10
Calculation:
Based on equation (1), the pressure (P) in each of the bulbs can de deduced by substituting the values of n in bulbs A, B and C and volume, V = 1.0 L
Now, pressure (P) is directly proportional to the number of moles (n). Therefore, under constant temperature, bulb C will have the highest pressure.
(b)
Interpretation:
The pressure in bulb C needs to be deduced if the pressure in bulb A is 0.500 atm.
Concept introduction:
The ideal gas equation is a thermodynamic equation of state which relates the pressure (P), volume (V), number of moles (n) and temperature (T) of an ideal gas through the following expression:
where R is the universal gas constant = 0.0821 L.atm/mol-K
Answer to Problem 86QAP
Pressure in Bulb C is 2.5 atm
Explanation of Solution
Given Information:
Volume (V) of bulb A = B = C = 1.0 L
Temperature (T) of bulb A = B = C
Pressure (P) in bulb A = 0.500 atm
Number of moles (n) of Ar gas in A = 2
Number of moles (n) of Ar gas in C = 10
Calculation:
Based on equation (1), the pressure (P) in bulbs A and C can be deduced by substituting the given values of n, V and P under constant T
(c)
Interpretation:
The total pressure needs to be deduced if the pressure in bulb A is 0.500 atm.
Concept introduction:
The ideal gas equation is a thermodynamic equation of state which relates the pressure (P), volume (V), number of moles (n) and temperature (T) of an ideal gas through the following expression:
where R is the universal gas constant = 0.0821 L.atm/mol-K
As per Dalton’s law, the total pressure exerted by a gas mixture is equal to the sum of the partial pressure of the individual gases.
Answer to Problem 86QAP
Total pressure = 4.00 atm
Explanation of Solution
Given Information:
Volume (V) of bulb A = B = C = 1.0 L
Temperature (T) of bulb A = B = C
Pressure (P) in bulb A = 0.500 atm
Number of moles (n) of Ar gas in A = 2
Number of moles (n) of Ar gas in B = 4
Number of moles (n) of Ar gas in C = 10
Calculation:
Based on equation (1), the pressure (P) in bulbs A and B can be deduced by substituting the given values of n, V and P under constant T
(d)
Interpretation:
The total pressure needs to be deduced after the valve between A and B is opened.
Concept introduction:
The ideal gas equation is a thermodynamic equation of state which relates the pressure (P), volume (V), number of moles (n) and temperature (T) of an ideal gas through the following expression:
where R is the universal gas constant = 0.0821 L.atm/mol-K
As per Dalton’s law, the total pressure exerted by a gas mixture is equal to the sum of the partial pressure of the individual gases.
Answer to Problem 86QAP
Total pressure after the valve between A and B is opened in 8.50 atm
Explanation of Solution
Given Information:
Volume (V) of bulb A = B = C = 1.0 L
Temperature (T) of bulb A = B = C
Pressure (P) in bulb A = 0.500 atm
Number of moles (n) of Ar gas in A = 2
Number of moles (n) of Ar gas in B = 4
Number of moles (n) of Ar gas in C = 10
Calculation:
When the valve between A and B is opened the Ar gas will diffuse from the region of high pressure i.e. bulb B to A until equilibrium is established.
Now the total number of moles (atoms) of Ar = 2 + 4 = 6. The final pressure in each bulb will be due to 6 moles (atoms) of Ar
Step 1: Calculate the final pressure in bulb A after mixing:
The initial pressure in bulb A = Pi = 0.500 atm
Initial moles of Ar gas in A = ni = 2
Final moles in A = nf = 6
The final pressure in bulb A = Pf
Under constant V and T, the ratio of the initial and final pressures would be:
Step 2: Calculate the final pressure in bulb B after mixing:
The initial pressure in bulb B = Pi = 1.50 atm
Initial moles of Ar gas in A = ni = 2
Final moles in A = nf = 6
The final pressure in bulb A = Pf
Under constant V and T, the ratio of the initial and final pressures would be:
Step 3: Calculate the total pressure after mixing:
The total pressure after the valve between A and B is opened is higher than that when the valve is closed.
(d)
Interpretation:
The total pressure needs to be deduced after the valve between B and C is opened.
Concept introduction:
The ideal gas equation is a thermodynamic equation of state which relates the pressure (P), volume (V), number of moles (n) and temperature (T) of an ideal gas through the following expression:
where R is the universal gas constant = 0.0821 L.atm/mol-K
As per Dalton’s law, the total pressure exerted by a gas mixture is equal to the sum of the partial pressure of the individual gases.
Answer to Problem 86QAP
Total pressure after the valve between B and C is opened in 9.25 atm
Explanation of Solution
Given Information:
Volume (V) of bulb A = B = C = 1.0 L
Temperature (T) of bulb A = B = C
Pressure (P) in bulb A = 0.500 atm
Number of moles (n) of Ar gas in A = 2
Number of moles (n) of Ar gas in B = 4
Number of moles (n) of Ar gas in C = 10
Calculation:
When the valve between B and C is opened the Ar gas will diffuse from the region of high pressure i.e. bulb C to B until equilibrium is established.
Now the total number of moles (atoms) of Ar = 4 + 10 = 14. The final pressure in each bulb will be due to 14 moles (atoms) of Ar
Step 1: Calculate the final pressure in bulb B after mixing:
The initial pressure in bulb B = Pi = 1.50 atm
Initial moles of Ar gas in B = ni = 4
Final moles in A = nf = 10
The final pressure in bulb B = Pf
Under constant V and T, the ratio of the initial and final pressures would be:
Step 2: Calculate the final pressure in bulb C after mixing:
The initial pressure in bulb C = Pi = 2.50 atm
Initial moles of Ar gas in C = ni = 10
Final moles in C = nf = 14
The final pressure in bulb A = Pf
Under constant V and T, the ratio of the initial and final pressures would be:
Step 3: Calculate the total pressure after mixing:
The total pressure after the valve between B and C is opened is higher than that when the valve is closed.
Want to see more full solutions like this?
Chapter 5 Solutions
EBK CHEMISTRY: PRINCIPLES AND REACTIONS
- 50 The first step in processing zinc metal from its ore, ZnS, is to react it with O2 according to the reaction 2ZnS(s)+3O2(g)2ZnO(s)+2SO2(g) If 620 kg of ZnS is to be reacted, what volume of oxygen at 0.977 atm 34.0 C is needed (at a minimum) to carry out this reaction?arrow_forwardLiquid oxygen was first prepared by heating potassium chlorate, KClO3, in a closed vessel to obtain oxygen at high pressure. The oxygen was cooled until it liquefied. 2KClO3(s)2KCl(s)+3O2(g) If 171 g of potassium chlorate reacts in a 2.70-L vessel, which was initially evacuated, what pressure of oxygen will be attained when the temperature is finally cooled to 25C? Use the preceding chemical equation and ignore the volume of solid product.arrow_forwardShown below are three containers of an ideal gas (A, B, and C), each equipped with a movable piston (assume that atmospheric pressure is 1.0 atm). a How do the pressures in these containers compare? b Are all the gases at the same temperature? If not, compare the temperatures. c If you cooled each of the containers in an ice-water bath to 0.0C, describe how the volumes and pressures of the gases in these containers would compare.arrow_forward
- You have an equimolar mixture of the gases SO2 and O2, along with some He, in a container fitted with a piston. The density of this mixture at STP is 1.924 g/L. Assume ideal behavior and constant temperature and pressure. a. What is the mole fraction of He in the original mixture? b. The SO2 and O2 react to completion to form SO3. What is the density of the gas mixture after the reaction is complete?arrow_forwardA 275-mL sample of CO gas is collected over water at 31C and 755 mmHg. If the temperature of the gas collection apparatus rises to 39C, what is the new volume of the sample? Assume that the barometric pressure does not change.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





