
Concept explainers
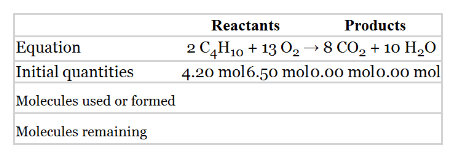
Completer the followin table using the given balanced equation and the initial quantities of reactants. Label the limiting reactant and the reactant used in excess.
| Reactants | Products | |
| Equation |
|
|
| Initial quantities | 4.20 mol 6.50 molo.00 molo.oo mol | |
| Molecules used or formed | ||
| Molecules remaining |
Interpretation:
The following table should be completed using the given information. The limiting reactant and the reactant used in excess should be predicted.
Concept Introduction:
Mole is the amount of the substance that contains the same number of particles or atoms or molecules. Molar mass is defined as average mass of atoms present in the chemical formula. It is the sum of the atomic masses of all the atoms present in the chemical formula of any compound.
Answer to Problem 88P
The limiting reactant is
Explanation of Solution
The given reaction is
Therefore, the moles of
Therefore, 4.20 moles of
The amount of product would be formed according to the
The amount of
The amount of
Therefore, the limiting reactant is
The limiting reactant is
Want to see more full solutions like this?
Chapter 5 Solutions
General, Organic, and Biological Chemistry - 4th edition
Additional Science Textbook Solutions
Principles of Chemistry: A Molecular Approach (3rd Edition)
Basic Chemistry (5th Edition)
Chemistry For Changing Times (14th Edition)
Chemistry: Matter and Change
- Ammonia can be formed by a direct reaction of nitrogen and hydrogen. N2(g) + 3 H2(g) 2 NH3(g) A tiny portion of the starting mixture is represented by the diagram, where the blue circles represent N and the white circles represent H. Which of these represents the product mixture? For the reaction of the given sample, which of these statements is true? (a) N2 is the limiting reactant. (b) H2 is the limiting reactant. (c) NH, is the limiting reactant. (d) No reactant is limiting: they are present in the correct stoichiometric ratio.arrow_forwardFor this reaction, fill in the table with the indicated quantities for the balanced equation. 4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2O(g)arrow_forward4.72 The picture shown depicts the species present at the start of a combustion reaction between methane, CH4 and oxygen, O2 (a) What is the limiting reactant? (b) Draw the resulting state after this set of reactants has reacted as far as possible.arrow_forward
- 4.8 In an experiment carried out at very low pressure, 13x1015 molecules of H2 are reacted with acetylene, C2H2, to form ethane, C2H6, on the surface of a catalyst. Write a balanced chemical equation for this reaction. How many molecules of acetylene are consumed?arrow_forwardFor the chemical reaction C3H8O2+4O23CO2+4H2O how many product molecules are formed when nine C3H8O2 molecules react?arrow_forward4.70 The particulate scale drawing shown depicts the products of a reaction between H2 and O2 molecules. (a) Draw a similar representation for the reactants that must have been present before the reaction took place. (b) Write a balanced chemical equation for the reaction, using the smallest possible whole number coefficients. (c) identify the limiting reactant, and explain how the pictures allow you to do so.arrow_forward
- Classify each of the following statements as true or false: a Coefficients in a chemical equation express the molar proportions among both reactants and products. b A stoichiometry problem can be solved with an unbalanced equation. c In solving a stoichiometry problem, the change from quantity of given substance to quantity of wanted substance is based on masses. d Percentage yield is actual yield expressed as a percentage of ideal yield. e The quantity of product of any reaction can be calculated only through the moles of the limiting reactant. f rH is positive for an endothermic reaction and negative for an exothermic reaction.arrow_forwardBalance the following chemical equation, and then answer the question below. :math>CuSO4(aq)+KI(s)CuI(s)+I2(s)+K2SO4(aq) ich reactant is the limiting reactant? Choose the best answer. th CuSO4and Kl are equally limiting because they react in a 1:1 mole ratio. i>CuSO4is the limiting reactant because only 2 moles are available compared with 4 moles of Kl. i>Kl is the limiting reactant because it is present in excess. i>Neither CuSO4 nor Kl is a limiting reactant because 6 total moles are present on the reactant side compared with 5 total moles on the product side. i>The limiting reactant cannot be determined because the starting amounts are not given.arrow_forwardSmall quantities of oxygen gas can be generated in the laboratory by the decomposition of hydrogen peroxide. The unbalanced equation for the reaction is H2O2(uz/)-? H2O(/) + O2(g) Calculate the mass of oxygen produced when 10.00 g of hydrogen peroxide decomposes.arrow_forward
- The pictures below show a molecular-scale view of a chemical reaction between H2 and CO to produce methanol, CH3OH The box on the left represents the reactants at the instant of mixing, and the box on the right shows what is left once the reaction has gone to completion. D Was there a limiting reactant in this reaction? If so, what was it? Write a balanced chemical equation for this reaction. As usual, your equation should use the smallest possible whole number coefficients for all substances.arrow_forwardFor each of the following unbalanced reactions, suppose exactly 5.00 g of each reactant is taken. Determine which reactant is limiting, and also determine what mass of the excess reagent will remain after the limiting reactant is consumed. Na2B4O7(s) + H2SO4(o H3BOj(j) + Na2SO4(u CaC,(s) + H2O(/) Ca(OH)2(s) + C2H2(g) NaCl(s) + H2SO4(/> HCl(g) + Na2SO4(s) SiO2(s) + C(x) —> Si(/) + CO(g)arrow_forward3.75 The following pictures show a molecular-scale view of a chemical reaction between the compounds AB2 and B2. (A atoms are shown in blue and B atoms in white). The box on the left represents the reactants at the instant of mixing, and the box on the right shows what is left once the reac- tion has gone to completion. Write a balanced chemical equation for this reaction. As usual, your equation should use the smallest possible whole number coefficients for all substances.arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





