
Concept explainers
(a)
The temperature of the steam after the flashing process and the power output from the turbine if the pressure of the steam at the exit of flash chamber is
(a)
Explanation of Solution
Given:
The inlet mass
The inlet pressure
The inlet temperature
The mass flow rate
The pressure at exit
The moisture content
Calculation:
Initially find out the properties of the geothermal water.
At state 1:
The geothermal water is extracted at the state of saturated liquid at the temperature of
The enthalpy at state 1 is as follows.
Refer Table A-4, “Saturated water-Temperature table”
The enthalpy
Refer Table A-1, “Molar mass, gas constant, and critical-point properties”.
At state 2:
The exit pressure of the flash chamber is
The geothermal steam is flashed at constant enthalpy. The exit steam of the flash chamber is at the quality of
Here, the fluid enthalpy is
Substitute
Refer Table A-5, “Saturated water-Pressure table”.
Obtain the following corresponding to the pressure of
At state 3:
There is no pressure drop in the separator. The separator separates vapor and liquid form the flashed steam, and the separated vapor alone sent to the turbine.
The enthalpy
Refer Table A-5, “Saturated water-Pressure table”.
The enthalpy
At state 4:
The steam is at the state of saturated mixture at the pressure of
The quality at state 4 is as follows.
The enthalpy
Refer Table A-5, “Saturated water-Pressure table”.
Obtain the following corresponding to the pressure of
The temperature of the steam after flashing process is equal to the saturation temperature at the exit pressure of flash chamber i.e.
Refer Table A-5, “Saturated water-Pressure table”.
The temperature
Thus, the exit temperature of flash chamber is
Write the formula for mass flow rate of vapor at entering the turbine.
Substitute
Draw schematic diagram of single flash geothermal power plant as shown in Figure 1.
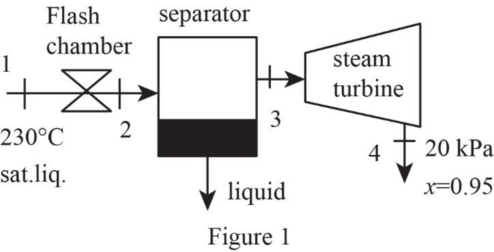
Write the general energy rate balance equation.
Here, the rate of total energy in is
Consider the system operates at steady state. Hence, the rate of change in net energy of the system becomes zero.
The Equation (I) is reduced as follows.
Refer Figure 1.
The flash chamber is nothing but the expansion valve. At expansion valve, the enthalpy kept constant.
Express the energy balance equation for the flash chamber.
Express the energy balance equation for the separator.
Express the energy balance equation for the turbine.
Substitute
Equation (III).
Thus, the power output turbine is
(b)
The temperature of the steam after the flashing process and the power output from the turbine if the pressure of the steam at the exit of flash chamber is
(b)
Explanation of Solution
Similarly for the pressure
At state 2:
The exit pressure of the flash chamber is
Refer Table A-5, “Saturated water-Pressure table”.
Obtain the following corresponding to the pressure of
At state 3:
There is no pressure drop in the separator. The separator separates vapor and liquid form the flashed steam, and the separated vapor alone sent to the turbine.
The enthalpy
Refer Table A-5, “Saturated water-Pressure table”.
The enthalpy
Calculation:
The temperature of the steam after flashing process is equal to the saturation temperature at the exit pressure of flash chamber i.e.
Refer Table A-5, “Saturated water-Pressure table”.
The temperature
Thus, the exit temperature of flash chamber is
Substitute
Substitute
Substitute
Equation (III).
Thus, the power output turbine is
(c)
The temperature of the steam after the flashing process and the power output from the turbine if the pressure of the steam at the exit of flash chamber is
(c)
Explanation of Solution
At state 2:
The exit pressure of the flash chamber is
Refer Table A-5, “Saturated water-Pressure table”.
Obtain the following corresponding to the pressure of
At state 3:
There is no pressure drop in the separator. The separator separates vapor and liquid form the flashed steam, and the separated vapor alone sent to the turbine.
The enthalpy
Refer Table A-5, “Saturated water-Pressure table”.
The enthalpy
The temperature of the steam after flashing process is equal to the saturation temperature at the exit pressure of flash chamber i.e.
Refer Table A-5, “Saturated water-Pressure table”.
The temperature
Thus, the exit temperature of flash chamber is
Substitute
Substitute
Substitute
Equation (III).
Thus, the power output turbine is
(d)
The temperature of the steam after the flashing process and the power output from the turbine if the pressure of the steam at the exit of flash chamber is
(d)
Explanation of Solution
At state 2:
The exit pressure of the flash chamber is
Refer Table A-5, “Saturated water-Pressure table”.
Obtain the following corresponding to the pressure of
At state 3:
There is no pressure drop in the separator. The separator separates vapor and liquid form the flashed steam, and the separated vapor alone sent to the turbine.
The enthalpy
Refer Table A-5, “Saturated water-Pressure table”.
The enthalpy
The temperature of the steam after flashing process is equal to the saturation temperature at the exit pressure of flash chamber i.e.
Refer Table A-5, “Saturated water-Pressure table”.
The temperature
Thus, the exit temperature of flash chamber is
Substitute
Substitute
Substitute
Equation (III).
Thus, the power output turbine is
Want to see more full solutions like this?
Chapter 6 Solutions
Fundamentals of Thermal-Fluid Sciences
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





