
(a)
Interpretation:
The Lewis structure for
Concept Introduction:
Lewis structure is also known as Lewis dot diagrams or electron dot structures. The bond between atoms and lone pairs of electrons that is present in the molecule. Lewis structure represents each atom and their position in structure using the chemical symbol. Excess electrons forms the lone pair are given by pair of dots, and are located next to the atom.
(a)
Explanation of Solution
Oxygen is in Group 6A and Chlorine is in Group 7A and the valence electrons present in the
The two chlorine atoms connect with one Oxygen atom through single bonds.
Chlorine atoms attain octet by adding six electrons as dots in pairs.
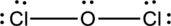
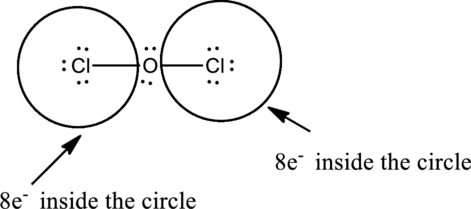
Complete the octet of the two chlorine atom uses
Put the last six electrons on Oxygen atom.
The correct Lewis structure of the
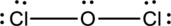
Oxygen has eight electrons four in the bonds and four as dots, hence the structure is complete.
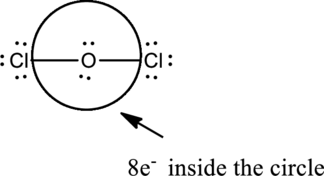
Hence, the total number of electrons can be counted as
(b)
Interpretation:
The Lewis structure for
Concept Introduction:
Refer part (a).
(b)
Explanation of Solution
Hydrogen atom is from Group one A and Oxygen atom is from group 6A, hence the valence electrons are
Complete the octet of the two chlorine atom uses
The incomplete Lewis structure of
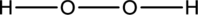
The correct Lewis structure of the

Hence, the total number of electrons can be counted as
(c)
Interpretation:
The Lewis structure for
Concept Introduction:
Refer part (a).
(c)
Explanation of Solution
The four Hydrogen atoms connect to boron with single bonds uses eight electrons. Boron atom is the central atom with the hydrogen atoms around it. So, the valence electron present in the
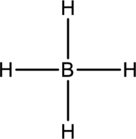
Boron atom must be the central atom with the four Hydrogen atoms bonded to it. Boron has eight electrons so, the structure is complete. Boron has eight electrons, and each Hydrogen atom has just two electrons, the structure clockwise, the total number of electrons can be counted
The correct Lewis structure of the
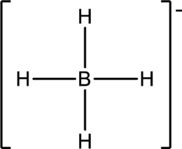
(d)
Interpretation:
The Lewis structure for
Concept Introduction:
Refer part (a).
(d)
Explanation of Solution
The four Hydrogen atoms connect to Phosphorous with single bonds uses eight electrons. Phosphorous atom is the central atom with the hydrogen atoms around it. So, the valence electrons present in the
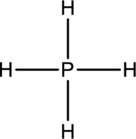
Phosphorous atom must be the central atom with the four Hydrogen atoms bonded to it. Phosphorous has eight electrons so, the structure is complete. It has eight electrons, and each Hydrogen atom has just two electrons, the structure clockwise, the total number of electrons can be counted
The structure is a
The correct Lewis structure of the
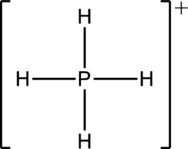
(e)
Interpretation:
The Lewis structure for
Concept Introduction:
Refer part (a).
(e)
Explanation of Solution
The five chlorine atoms connect to Phosphorous with single bonds uses ten electrons. Phosphorous atom is the central atom with the Chlorine atoms around it.
The number of valence electrons present in
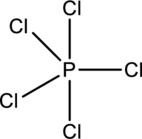
Chlorine atoms prefer making only one bond, and Phosphorous prefers to make three and five bonds. So use Phosphorous atom as central atom with the five Chlorine atoms around it.
Each Chlorine atom has three lone pair and one bond pair so it attains octet.
The correct Lewis structure of the
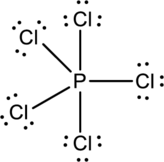
The total number of electrons can be counted as
Want to see more full solutions like this?
Chapter 6 Solutions
Chemistry: The Molecular Science, Hybrid Edition (with OWLv2 24-Months Printed Access Card)
- Write a Lewis structure for the amide ion, NH2─, and assign formal charges to each atom.arrow_forwardHow many resonance structures can be drawn for the hydrogen tellurate ion (HTeO4–) in which the central tellurium atom bears a –1 formal charge and the oxygens bear formal charges of either zero or –1? Enter your answer as a whole number.arrow_forwardDraw the Lewis structures of cyanate (OCN-) and fulminate (CNO-) ions and calculate their formal charges. Discuss their stability by giving resonance structures.arrow_forward
- Determine the number of valence electrons in sulfuric acid (H₂SO₄) and then draw the corresponding Lewis structure (with minimized formal charges).arrow_forwardWhat is the formal charge on the chlorine (Cl) atom in PCl₆⁻?arrow_forwardBased on average bond enthalpies, would you expect a photon capable ofdissociating a C¬Cl bond to have sufficient energy to dissociate a C¬Br bond?arrow_forward
- Draw the Lewis structure of the azide ion, N3, and calculate the formal charge on each nitrogen atom in the structure:arrow_forwardIn developing a Lewis structure for NO +, the nitroxonium ion, how many valence electrons must you account for?arrow_forwardHow many double bonds can be found in the best Lewis stucture of COF 2?arrow_forward
- What is the formal charge on the central chlorine atom in the molecular ion [ClO4]- ? Assume that all of the Cl-O bonds are single bonds.arrow_forwardDraw Lewis structures for these ions and show which atom in each bears the formal charge. Q.) H3O+arrow_forwardCalculate the following: Standard enthalpy of formation of methane, the enthalpy of atomization of methane and the Bond energy term for the C-H bond.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

