
(a)
Interpretation:
The bond type for each bond in
Concept Introduction:
Lewis structure is also known as Lewis dot diagrams or electron dot structures. The bond between atoms and lone pairs of electrons that is present in the molecule. Lewis structure represents each atom and their position in structure using the chemical symbol. Excess electrons forms the lone pair are given by pair of dots, and are located next to the atom.
The formula for the formal charge can be written as
(a)
Explanation of Solution
The correct Lewis structure of the
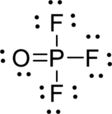
Draw a chart:
The total number of valence electron is
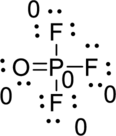
The phosphorous atom has three single bonds and one double bond; both fluorine and oxygen have a one single bond. All atoms in
The bond between
(b)
Interpretation:
The bond type for each bond in
Concept Introduction:
Refer part (a).
(b)
Explanation of Solution
The correct Lewis structure of the
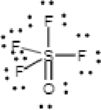
Draw a chart:
The total number of valence electron is
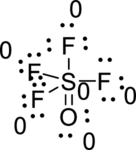
The sulfur atom has four single bonds and one double bond; all fluorine has a one single bond, oxygen has double bond. All atoms in
The bond between
Want to see more full solutions like this?
Chapter 6 Solutions
Chemistry: The Molecular Science, Hybrid Edition (with OWLv2 24-Months Printed Access Card)
- Nitrosyl azide, N4O, is a pale yellow solid first synthesized in 1993. Write the Lewis structure for nitrosyl azide.arrow_forwardWrite reasonable Lewis structures for the following species, none of which follow the octet rule. (a) BF3 (b) NO (c) CO+ (d) ClO3arrow_forwardChemical species are said to be isoelectronic if they have the same Lewis structure (regardless of charge). Consider these ions and write a Lewis structure for a neutral molecule that is isoelectronic with them. (a) CN–, (b) NH4+ (c) CO3 2–arrow_forward
- provide the following information including the lewis structure and the 3d sketch for SeF4 and BrF5arrow_forwardWhy is it that Xe, a noble gas element we normally think of as being unreactive can form covalent compounds?arrow_forwardAluminum oxide (Al₂ O₃) is a widely used industrial abrasive(emery, corundum), for which the specific application depends onthe hardness of the crystal. What does this hardness imply about the magnitude of the lattice energy? Would you have predictedfrom the chemical formula that Al₂ O₃ is hard? Explain.arrow_forward
- What is the formal charge on carbon in COCl2?arrow_forwardDraw the Lewis structure of N2O5 with minimized formal chargesarrow_forwardChloral, Cl3C—CH=O, reacts with water to form the sedative and hypnotic agent chloral hydrate, Cl3C—CH(OH)2. Draw Lewis structures for these substances, and describe the change in molecular shape, if any, that occurs around each of the carbon atoms during the reaction.arrow_forward
- The halogens form a class of compounds called the interhalogens, in which halogen atoms covalently bond to each other. Write the Lewis structures for the interhalogens BrCl3 and ICl4−.arrow_forwardPhosgene, a substance used in poisonous gas warfare during World War I, is so named because it was first prepared by the action of sunlight on a mixture of carbon monoxide and chlorine gases. Its name comes from the Greek words phos (light) and genes (born of). Phosgene has the following elemental composition: 12.14% C, 16.17% O, and 71.69% Cl by mass. Its molar mass is 98.9 g/mol. (d) Using average bond enthalpies, estimate H for the formation of gaseous phosgene from CO(g) and Cl2(g).arrow_forwardWhat is the formal charge on each atom in HNO3 and the Lewis structure for it?arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning


