
Interpretation:
Similarity and dissimilarity between solid and liquid should be identified.
Concept introduction:
The liquid can be compressed and have loosely packed molecules so that it cannot retain a shape in comparison to solid in which molecules are tightly packed to give it a specific shape. Hence, solid and liquid have some similarities and dissimilarities as well.
Answer to Problem 1RQ
Solution:
Liquid and solid have fixed volume and can change their state when subjected to heat.
In solid the molecules are tightly packed whereas liquid having loosely packed molecules but both of them are present in the condensed phase.
Explanation of Solution
Liquid and solid have fixed volume and can change their state when subjected to heat.
Solid and liquid are present in the condensed phase or their molecules are closer in comparison to gaseous in which molecules are far apart.
In solid, the molecules are tightly packed with each other. The molecules in solid are so closely packed so that their movement is so reduced to give it a confined structure of solidity whereas liquid having loosely packed molecules which cannot give it a perfect shape.
The molecules of solid and liquid can be represented as follows:
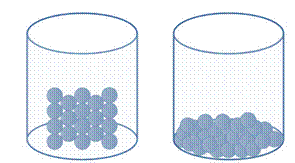
Solid Liquid
Hence, in solid the molecules are tightly packed whereas liquid having loosely packed molecules but both of them are present in the condensed phase.
Want to see more full solutions like this?
Chapter 6 Solutions
Chemistry for Changing Times, Books a la Carte Edition (14th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





